Analysis of Diffusion in Solid-State Electrolytes through MD Simulations, Improvement of the Li-Ion Conductivity in β-Li3PS4 as an Example
- PMID: 30057999
- PMCID: PMC6058286
- DOI: 10.1021/acsaem.8b00457
Analysis of Diffusion in Solid-State Electrolytes through MD Simulations, Improvement of the Li-Ion Conductivity in β-Li3PS4 as an Example
Abstract
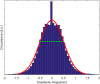
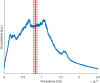
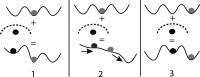
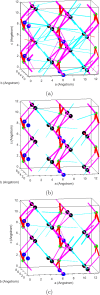
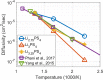
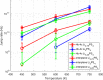
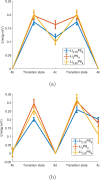
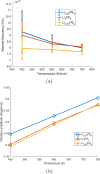
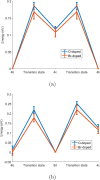
Molecular dynamics simulations are a powerful tool to study diffusion processes in battery electrolyte and electrode materials. From molecular dynamics simulations, many properties relevant to diffusion can be obtained, including the diffusion path, amplitude of vibrations, jump rates, radial distribution functions, and collective diffusion processes. Here it is shown how the activation energies of different jumps and the attempt frequency can be obtained from a single molecular dynamics simulation. These detailed diffusion properties provide a thorough understanding of diffusion in solid electrolytes, and provide direction for the design of improved solid electrolyte materials. The presently developed analysis methodology is applied to DFT MD simulations of Li-ion diffusion in β-Li3PS4. The methodology presented is generally applicable to diffusion in crystalline materials and facilitates the analysis of molecular dynamics simulations. The code used for the analysis is freely available at: https://bitbucket.org/niekdeklerk/md-analysis-with-matlab. The results on β-Li3PS4 demonstrate that jumps between bc planes limit the conductivity of this important class of solid electrolyte materials. The simulations indicate that the rate-limiting jump process can be accelerated significantly by adding Li interstitials or Li vacancies, promoting three-dimensional diffusion, which results in increased macroscopic Li-ion diffusivity. Li vacancies can be introduced through Br doping, which is predicted to result in an order of magnitude larger Li-ion conductivity in β-Li3PS4. Furthermore, the present simulations rationalize the improved Li-ion diffusivity upon O doping through the change in Li distribution in the crystal. Thus, it is demonstrated how a thorough understanding of diffusion, based on thorough analysis of MD simulations, helps to gain insight and develop strategies to improve the ionic conductivity of solid electrolytes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Bauer C.; Hofer J.; Althaus H.-J.; Del Duce A.; Simons A. The Environmental Performance of Current and Future Passenger Vehicles: Life Cycle Assessment Based on a Novel Scenario Analysis Framework. Appl. Energy 2015, 157, 871–883. 10.1016/j.apenergy.2015.01.019. - DOI
-
- Lotsch B. V.; Maier J. Relevance of Solid Electrolytes for Lithium-Based Batteries: A Realistic View. J. Electroceram. 2017, 38, 128–141. 10.1007/s10832-017-0091-0. - DOI
-
- Placke T.; Kloepsch R.; Dühnen S.; Winter M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: the Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. 10.1007/s10008-017-3610-7. - DOI
-
- Lu X. C.; Xia G. G.; Lemmon J. P.; Yang Z. G. Advanced Materials for Sodium-Beta Alumina Batteries: Status, Challenges and Perspectives. J. Power Sources 2010, 195, 2431–2442. 10.1016/j.jpowsour.2009.11.120. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
