The KHENERGY Study: Safety and Efficacy of KH176 in Mitochondrial m.3243A>G Spectrum Disorders
- PMID: 30058726
- PMCID: PMC6704357
- DOI: 10.1002/cpt.1197
The KHENERGY Study: Safety and Efficacy of KH176 in Mitochondrial m.3243A>G Spectrum Disorders
Abstract
KH176 is a potent intracellular reduction-oxidation-modulating compound developed to treat mitochondrial disease. We studied tolerability, safety, pharmacokinetics, pharmacodynamics, and efficacy of twice daily oral 100 mg KH176 for 28 days in a double-blind, randomized, placebo-controlled, two-way crossover phase IIA study in 18 adult m.3243A>G patients without cardiovascular involvement. Efficacy parameters included clinical and functional outcome measures and biomarkers. The trial was registered within ClinicalTrials.gov (NCT02909400), the European Clinical Trials Database (2016-001696-79), and ISRCTN (43372293) (The KHENERGY study). Twice daily oral 100 mg KH176 was well tolerated and appeared safe. No serious treatment-emergent adverse events were reported. No significant improvements in gait parameters or other outcome measures were obtained, except for a positive effect on alertness and mood, although a coincidence due to multiplicity cannot be ignored. The results of the study provide first data on safety and efficacy of KH176 in patients with mitochondrial disease and will be instrumental in designing future clinical trials.
© 2018 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
E.S. is the chief marketing officer, J.B. is the chief operating officer, and J.S. is the chief executive officer of Khondrion BV. All other authors report no disclosures.
Figures
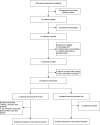

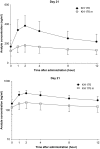
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

