α1-FANGs: Protein Ligands Selective for the α-Bungarotoxin Site of the α1-Nicotinic Acetylcholine Receptor
- PMID: 30059207
- PMCID: PMC8763392
- DOI: 10.1021/acschembio.8b00513
α1-FANGs: Protein Ligands Selective for the α-Bungarotoxin Site of the α1-Nicotinic Acetylcholine Receptor
Abstract
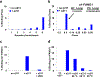
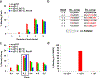
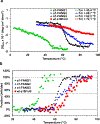
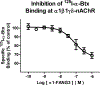
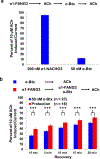
Nicotinic acetylcholine receptors (nAChRs) are pentameric ligand-gated ion channels that play a central role in neuronal and neuromuscular signal transduction. Here, we have developed FANG ligands, fibronectin antibody-mimetic nicotinic acetylcholine receptor-generated ligands, using mRNA display. We generated a 1 trillion-member primary e10FnIII library to target a stabilized α1 nicotinic subunit (α211). This library yielded 270000 independent potential protein binding ligands. The lead sequence, α1-FANG1, represented 25% of all library sequences, showed the highest-affinity binding, and competed with α-bungarotoxin (α-Btx). To improve this clone, a new library based on α1-FANG1 was subjected to heat, protease, binding, off-rate selective pressures, and point mutations. This resulted in α1-FANG2 and α1-FANG3. These proteins bind α211 with KD values of 3.5 nM and 670 pM, respectively, compete with α-Btx, and show improved subunit specificity. α1-FANG3 is thermostable ( Tm = 62 °C) with a 6 kcal/mol improvement in folding free energy compared with that of the parent α1-FANG1. α1-FANG3 competes directly with the α-Btx binding site of intact neuromuscular heteropentamers [(α1)2β1γδ] in mammalian culture-derived cellular membranes and in Xenopus laevis oocytes expressing these nAChRs. This work demonstrates that mRNA display against a monomeric ecto-domain of a pentamer has the capability to select ligands that bind that subunit in both a monomeric and a pentameric context. Overall, our work provides a route to creating a new family of stable, well-behaved proteins that specifically target this important receptor family.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
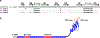
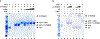
References
-
- Lester HA, Dibas MI, Dahan DS, Leite JF, and Dougherty DA (2004) Cys-loop receptors: new twists and turns. Trends Neurosci. 27, 329–336. - PubMed
-
- Sine SM, and Engel AG (2006) Recent advances in Cys-loop receptor structure and function. Nature 440, 448–455. - PubMed
-
- Corringer PJ, Le Novère N, and Changeux JP (2000) Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 40, 431–458. - PubMed
-
- Kasheverov IE, Utkin YN, and Tsetlin VI (2009) Naturally occurring and synthetic peptides acting on nicotinic acetylcholine receptors. Curr. Pharm. Des. 15, 2430–2452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

