Cryopreservation of human mucosal tissues
- PMID: 30059507
- PMCID: PMC6066204
- DOI: 10.1371/journal.pone.0200653
Cryopreservation of human mucosal tissues
Abstract
Background: Cryopreservation of leukocytes isolated from the cervicovaginal and colorectal mucosa is useful for the study of cellular immunity (see Hughes SM et al. PLOS ONE 2016). However, some questions about mucosal biology and sexually transmitted infections are better addressed with intact mucosal tissue, for which there is no standard cryopreservation protocol.
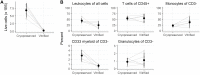
Methods and findings: To find an optimal preservation protocol for mucosal tissues, we tested slow cooling (1°C/min) with 10% dimethylsulfoxide (designated "cryopreservation") and fast cooling (plunge in liquid nitrogen) with 20% dimethylsulfoxide and 20% ethylene glycol ("vitrification"). We compared fresh and preserved human cervicovaginal and colorectal tissues in a range of assays, including metabolic activity, human immunodeficiency virus infection, cell phenotype, tissue structure by hematoxylin-and-eosin staining, cell number and viability, production of cytokines, and microbicide drug concentrations. Metabolic activity, HIV infectability, and tissue structure were similar in cryopreserved and vitrified vaginal tissues. However, vitrification led to poor cell recovery from the colorectal mucosa, with 90% fewer cells recovered after isolation from vitrified colorectal tissues than from cryopreserved. HIV infection rates were similar for fresh and cryopreserved ectocervical tissues, whereas cryopreserved colorectal tissues were less easily infected than fresh tissues (hazard ratio 0.7 [95% confidence interval 0.4, 1.2]). Finally, we compared isolation of cells before and after cryopreservation. Cell recoveries were higher when cells were isolated after freezing and thawing (71% [59-84%]) than before (50% [38-62%]). Cellular function was similar to fresh tissue in both cases. Microbicide drug concentrations were lower in cryopreserved explants compared to fresh ones.
Conclusions: Cryopreservation of intact cervicovaginal and colorectal tissues with dimethylsulfoxide works well in a range of assays, while the utility of vitrification is more limited. Cell yields are higher from cryopreserved intact tissue pieces than from thawed cryopreserved single cell suspensions isolated before freezing, but T cell functions are similar.
Conflict of interest statement
Merck & Co., Inc., Kenilworth, NJ USA provided MK-2018 for use in this study. The authors declare that no other competing interests exist and confirm that this does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures







References
-
- Dezzutti CS, Richardson-Harman N, Rohan LC, Marzinke MA, Hoesley CJ, Panther L, et al. Pharmacodynamic correlations using fresh and cryopreserved tissue following use of vaginal rings containing dapivirine and/or maraviroc in a randomized, placebo controlled trial. Medicine (Baltimore). 2016;95: e4174 10.1097/MD.0000000000004174 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

