Harmful Effects and Potential Benefits of Anti-Tumor Necrosis Factor (TNF)-α on the Liver
- PMID: 30060508
- PMCID: PMC6121684
- DOI: 10.3390/ijms19082199
Harmful Effects and Potential Benefits of Anti-Tumor Necrosis Factor (TNF)-α on the Liver
Abstract
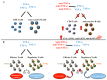
Anti-tumor necrosis factor (TNF)-α agents represent an effective treatment for chronic inflammatory diseases. However, some concerns about their potentially undesirable effects on liver function have been reported. On the other hand, evidence of their therapeutic effects on certain liver diseases is accumulating. Many data showed the safety of anti-TNF-α in patients with chronic hepatitis B and C and in liver transplanted patients even if a strict follow-up and prophylaxis are recommended in well-defined subgroups. On the other side, anti-TNF-α-induced liver injury is not a rare event. However, it is often reversible after anti-TNF-α withdrawal. Anti-TNF-α agents have been tested in advanced stages of severe alcoholic hepatitis and non-alcoholic fatty liver disease. Limited data on the efficacy of anti-TNF-α in patients with autoimmune hepatitis and primary biliary cholangitis are also available. In this review, we explored the hepatic safety concerns in patients receiving anti-TNF-α agents with and without pre-existent hepatic diseases. In addition, the available evidence on their potential benefits in the treatment of specific hepatic diseases is discussed.
Keywords: adalimumab; alcoholic hepatitis; autoimmune hepatitis; certolizumab pegol; drug-induced liver injury; etanercept; golimumab; hepatitis B virus; hepatitis C virus; infliximab; non-alcoholic fatty liver disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nesbitt A., Fossati G., Bergin M., Stephens P., Stephens S., Foulkes R., Brown D., Robinson M., Bourne T. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm. Bowel Dis. 2007;13:1323–1332. doi: 10.1002/ibd.20225. - DOI - PubMed
-
- Rossi R.E., Parisi I., Despott E.J., Burroughs A.K., O’Beirne J., Conte D., Hamilton M.I., Murray C.D. Anti-tumour necrosis factor agent and liver injury: Literature review, recommendations for management. World J. Gastroenterol. 2014;20:17352–17359. doi: 10.3748/wjg.v20.i46.17352. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

