Novel Neohesperidin Dihydrochalcone Analogue Inhibits Adipogenic Differentiation of Human Adipose-Derived Stem Cells through the Nrf2 Pathway
- PMID: 30060630
- PMCID: PMC6121477
- DOI: 10.3390/ijms19082215
Novel Neohesperidin Dihydrochalcone Analogue Inhibits Adipogenic Differentiation of Human Adipose-Derived Stem Cells through the Nrf2 Pathway
Abstract
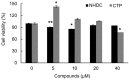
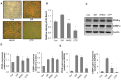
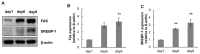
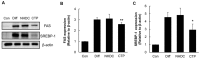
Obesity, characterized by excess lipid accumulation, has emerged as a leading public health problem. Excessive, adipocyte-induced lipid accumulation raises the risk of metabolic disorders. Adipose-derived stem cells (ASCs) are mesenchymal stem cells (MSCs) that can be obtained from abundant adipose tissue. High fat mass could be caused by an increase in the size (hypertrophy) and number (hyperplasia) of adipocytes. Reactive oxygen species (ROS) are involved in the adipogenic differentiation of human adipose-derived stem cells (hASCs). Lowering the level of ROS is important to blocking or retarding the adipogenic differentiation of hASCs. Nuclear factor erythroid 2-related factor-2 (Nrf2) is a transcription factor that mediates various antioxidant enzymes and regulates cellular ROS levels. Neohesperidin dihydrochalcone (NHDC), widely used as artificial sweetener, has been shown to have significant free radical scavenging activity. In the present study, (E)-3-(4-chlorophenyl)-1-(2,4,6-trimethoxyphenyl)prop-2-en-1-one (CTP), a novel NHDC analogue, was synthesized and examined to determine whether it could inhibit adipogenic differentiation. The inhibition of adipogenic differentiation in hASCs was tested using NHDC and CTP. In the CTP group, reduced Oil Red O staining was observed compared with the differentiation group. CTP treatment also downregulated the expression of PPAR-γ and C/EBP-α, adipogenic differentiation markers in hASCs, compared to the adipogenic differentiation group. The expression of FAS and SREBP-1 decreased in the CTP group, along with the fluorescent intensity (amount) of ROS. Expression of the Nrf2 protein was slightly decreased in the differentiation group. Meanwhile, in both the NHDC and CTP groups, Nrf2 expression was restored to the level of the control group. Moreover, the expression of HO-1 and NQO-1 increased significantly in the CTP group. Taken together, these results suggest that CTP treatment suppresses the adipogenic differentiation of hASCs by decreasing intracellular ROS, possibly through activation of the Nrf2 cytoprotective pathway. Thus, the use of bioactive substances such as CTP, which activates Nrf2 to reduce the cellular level of ROS and inhibit the adipogenic differentiation of hASCs, could be a new strategy for overcoming obesity.
Keywords: Nrf2; adipogenic differentiation; adipose stem cells; neohesperidin dihydrochalcone; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

