MicroRNA-218-5p Promotes Endovascular Trophoblast Differentiation and Spiral Artery Remodeling
- PMID: 30061037
- PMCID: PMC6127878
- DOI: 10.1016/j.ymthe.2018.07.009
MicroRNA-218-5p Promotes Endovascular Trophoblast Differentiation and Spiral Artery Remodeling
Abstract
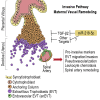
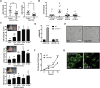
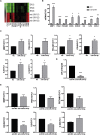
Preeclampsia (PE) is the leading cause of maternal and neonatal morbidity and mortality. Defects in trophoblast invasion, differentiation of endovascular extravillous trophoblasts (enEVTs), and spiral artery remodeling are key factors in PE development. There are no markers clinically available to predict PE, leaving expedited delivery as the only effective therapy. Dysregulation of miRNA in clinical tissues and maternal circulation have opened a new avenue for biomarker discovery. In this study, we investigated the role of miR-218-5p in PE development. miR-218-5p was highly expressed in EVTs and significantly downregulated in PE placentas. Using first-trimester trophoblast cell lines and human placental explants, we found that miR-218-5p overexpression promoted, whereas anti-miR-218-5p suppressed, trophoblast invasion, EVT outgrowth, and enEVT differentiation. Furthermore, miR-218-5p accelerated spiral artery remodeling in a decidua-placenta co-culture. The effect of miR-218-5p was mediated by the suppression of transforming growth factor (TGF)-β2 signaling. Silencing of TGFB2 mimicked, whereas treatment with TGF-β2 partially reversed, the effects of miR-218-5p. Taken together, these findings demonstrate that miR-218-5p promotes trophoblast invasion and enEVT differentiation through a novel miR-218-5p-TGF-β2 pathway. This study elucidates the role of an miRNA in enEVT differentiation and spiral artery remodeling and suggests that downregulation of miR-218-5p contributes to PE development.
Keywords: TGF-β; endovascular trophoblasts; miR-218-5p; micorRNA; placenta-decidua co-culture; preeclampsia; remodeling; spiral artery; vascular transformation.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009;33:130–137. - PubMed
-
- Davis E.F., Lazdam M., Lewandowski A.J., Worton S.A., Kelly B., Kenworthy Y., Adwani S., Wilkinson A.R., McCormick K., Sargent I. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

