Ceftazidime-Avibactam Susceptibility Breakpoints against Enterobacteriaceae and Pseudomonas aeruginosa
- PMID: 30061279
- PMCID: PMC6201065
- DOI: 10.1128/AAC.02590-17
Ceftazidime-Avibactam Susceptibility Breakpoints against Enterobacteriaceae and Pseudomonas aeruginosa
Abstract
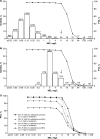
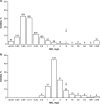
Clinical susceptibility breakpoints against Enterobacteriaceae and Pseudomonas aeruginosa for the ceftazidime-avibactam dosage regimen of 2,000/500 mg every 8 h (q8h) by 2-h intravenous infusion (adjusted for renal function) have been established by the FDA, CLSI, and EUCAST as susceptible (MIC, ≤8 mg/liter) and resistant (MIC, >8 mg/liter). The key supportive data from pharmacokinetic/pharmacodynamic analyses, in vitro surveillance, including molecular understanding of relevant resistance mechanisms, and efficacy in regulatory clinical trials are collated and analyzed here.
Trial registration: ClinicalTrials.gov NCT00752219 NCT01499290 NCT01500239 NCT01726023 NCT01595438 NCT01599806 NCT01644643 NCT01808092.
Keywords: MIC breakpoints; ceftazidime-avibactam.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- de Jonge BL, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. 2016. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae Isolates collected during the INFORM global surveillance study (2012 to (2014). Antimicrob Agents Chemother 60:3163–3169. doi:10.1128/AAC.03042-15. - DOI - PMC - PubMed
-
- Karlowsky JA, Biedenbach DJ, Kazmierczak KM, Stone GG, Sahm DF. 2016. Activity of ceftazidime-avibactam against extended-spectrum- and AmpC beta-lactamase-producing Enterobacteriaceae collected in the INFORM global surveillance study from 2012 to 2014. Antimicrob Agents Chemother 60:2849–2857. doi:10.1128/AAC.02286-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

