Cancer modeling by Transgene Electroporation in Adult Zebrafish (TEAZ)
- PMID: 30061297
- PMCID: PMC6177007
- DOI: 10.1242/dmm.034561
Cancer modeling by Transgene Electroporation in Adult Zebrafish (TEAZ)
Abstract
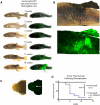
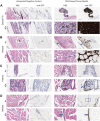
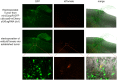
Transgenic animals are invaluable for modeling cancer genomics, but often require complex crosses of multiple germline alleles to obtain the desired combinations. Zebrafish models have advantages in that transgenes can be rapidly tested by mosaic expression, but typically lack spatial and temporal control of tumor onset, which limits their utility for the study of tumor progression and metastasis. To overcome these limitations, we have developed a method referred to as Transgene Electroporation in Adult Zebrafish (TEAZ). TEAZ can deliver DNA constructs with promoter elements of interest to drive fluorophores, oncogenes or CRISPR-Cas9-based mutagenic cassettes in specific cell types. Using TEAZ, we created a highly aggressive melanoma model via Cas9-mediated inactivation of Rb1 in the context of BRAFV600E in spatially constrained melanocytes. Unlike prior models that take ∼4 months to develop, we found that TEAZ leads to tumor onset in ∼7 weeks, and these tumors develop in fully immunocompetent animals. As the resulting tumors initiated at highly defined locations, we could track their progression via fluorescence, and documented deep invasion into tissues and metastatic deposits. TEAZ can be deployed to other tissues and cell types, such as the heart, with the use of suitable transgenic promoters. The versatility of TEAZ makes it widely accessible for rapid modeling of somatic gene alterations and cancer progression at a scale not achievable in other in vivo systems.
Keywords: Cancer; Electroporation; Melanoma; Metastasis; Zebrafish.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Balciunas D., Wangensteen K. J., Wilber A., Bell J., Geurts A., Sivasubbu S., Wang X., Hackett P. B., Largaespada D. A., McIvor R. S. et al. (2006). Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2, e169 10.1371/journal.pgen.0020169 - DOI - PMC - PubMed
-
- Berghmans S., Murphey R. D., Wienholds E., Neuberg D., Kutok J. L., Fletcher C. D. M., Morris J. P., Liu T. X., Schulte-Merker S., Kanki J. P. et al. (2005). tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 102, 407-412. 10.1073/pnas.0406252102 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

