Identification of a biologically active fragment of ALK and LTK-Ligand 2 (augmentor-α)
- PMID: 30061385
- PMCID: PMC6099872
- DOI: 10.1073/pnas.1807881115
Identification of a biologically active fragment of ALK and LTK-Ligand 2 (augmentor-α)
Abstract
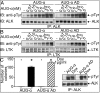
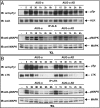
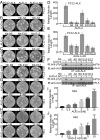
Elucidating the physiological roles and modes of action of the recently discovered ligands (designated ALKAL1,2 or AUG-α,β) of the receptor tyrosine kinases Anaplastic Lymphoma Kinase (ALK) and Leukocyte Tyrosine Kinase (LTK) has been limited by difficulties in producing sufficient amounts of the two ligands and their poor stability. Here we describe procedures for expression and purification of AUG-α and a deletion mutant lacking the N-terminal variable region. Detailed biochemical characterization of AUG-α by mass spectrometry shows that the four conserved cysteines located in the augmentor domain (AD) form two intramolecular disulfide bridges while a fifth, primate-specific cysteine located in the N-terminal variable region mediates dimerization through formation of a disulfide bridge between two AUG-α molecules. In contrast to AUG-α, the capacity of AUG-α AD to undergo dimerization is strongly compromised. However, full-length AUG-α and the AUG-α AD deletion mutant stimulate similar tyrosine phosphorylation of cells expressing either ALK or LTK. Both AUG-α and AUG-α AD also stimulate a similar profile of MAP kinase response in L6 cells and colony formation in soft agar by autocrine stimulation of NIH 3T3 cells expressing ALK. Moreover, both AUG-α and AUG-α AD stimulate neuronal differentiation of human neuroblastoma NB1 and PC12 cells in a similar dose-dependent manner. Taken together, these experiments show that deletion of the N-terminal variable region minimally affects the activity of AUG-α toward LTK or ALK stimulation in cultured cells. Reduced dimerization might be compensated by high local concentration of AUG-α AD bound to ALK at the cell membrane and by potential ligand-induced receptor-receptor interactions.
Keywords: active fragment; cell signaling; cytokine; receptor activation; receptor tyrosine kinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Murray PB, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8:ra6. - PubMed
-
- Ben-Neriah Y, Bauskin AR. Leukocytes express a novel gene encoding a putative transmembrane protein-kinase devoid of an extracellular domain. Nature. 1988;333:672–676. - PubMed
-
- Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

