Overexpression of endophilin A1 exacerbates synaptic alterations in a mouse model of Alzheimer's disease
- PMID: 30061577
- PMCID: PMC6065365
- DOI: 10.1038/s41467-018-04389-0
Overexpression of endophilin A1 exacerbates synaptic alterations in a mouse model of Alzheimer's disease
Abstract
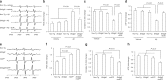
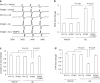
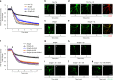
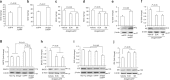
Endophilin A1 (EP) is a protein enriched in synaptic terminals that has been linked to Alzheimer's disease (AD). Previous in vitro studies have shown that EP can bind to a variety of proteins, which elicit changes in synaptic transmission of neurotransmitters and spine formation. Additionally, we previously showed that EP protein levels are elevated in AD patients and AD transgenic animal models. Here, we establish the in vivo consequences of upregulation of EP expression in amyloid-β peptide (Aβ)-rich environments, leading to changes in both long-term potentiation and learning and memory of transgenic animals. Specifically, increasing EP augmented cerebral Aβ accumulation. EP-mediated signal transduction via reactive oxygen species (ROS)/p38 mitogen-activated protein (MAP) kinase contributes to Aβ-induced mitochondrial dysfunction, synaptic injury, and cognitive decline, which could be rescued by blocking either ROS or p38 MAP kinase activity.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Scheff SW, Sparks DL, Price DA. Quantitative assessment of synaptic density in the outer molecular layer of the hippocampal dentate gyrus in Alzheimer’s disease. Dementia. 1996;7:226–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

