Hydrogenation catalyst generates cyclic peptide stereocentres in sequence
- PMID: 30061616
- PMCID: PMC6824594
- DOI: 10.1038/s41557-018-0089-5
Hydrogenation catalyst generates cyclic peptide stereocentres in sequence
Abstract
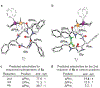
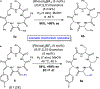
Molecular recognition plays a key role in enzyme-substrate specificity, the regulation of genes, and the treatment of diseases. Inspired by the power of molecular recognition in enzymatic processes, we sought to exploit its use in organic synthesis. Here we demonstrate how a synthetic rhodium-based catalyst can selectively bind a dehydroamino acid residue to initiate a sequential and stereoselective synthesis of cyclic peptides. Our combined experimental and theoretical study reveals the underpinnings of a cascade reduction that occurs with high stereocontrol and in one direction around a macrocyclic ring. As the catalyst can dissociate from the peptide, the C to N directionality of the hydrogenation reactions is controlled by catalyst-substrate recognition rather than a processive mechanism in which the catalyst remains bound to the macrocycle. This mechanistic insight provides a foundation for the use of cascade hydrogenations.
Figures

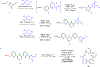
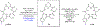

References
-
- Rebek J. Molecular recognition with model systems. Angew. Chem. Int. Ed 29, 245–255 (1990).
-
- Milton RC, Milton SC & Kent SB Total chemical synthesis of a D-enzyme: the enantiomers of HIV-1 protease show reciprocal chiral substrate specificity. Science 256, 1445–1448 (1992). - PubMed
-
- Koeller KM & Wong C-H Enzymes for chemical synthesis. Nature 409, 232–240 (2001). - PubMed
-
- Steitz TA From the structure and function of the ribosome to new antibiotics. Angew. Chem. Int. Ed 49, 4381–4398 (2010). - PubMed
-
- Ramakrishnan V. Unraveling the structure of the ribosome. Angew. Chem. Int. Ed 49, 4355–4380 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

