Molecular therapies and precision medicine for hepatocellular carcinoma
- PMID: 30061739
- PMCID: PMC12452113
- DOI: 10.1038/s41571-018-0073-4
Molecular therapies and precision medicine for hepatocellular carcinoma
Abstract
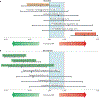
The global burden of hepatocellular carcinoma (HCC) is increasing and might soon surpass an annual incidence of 1 million cases. Genomic studies have established the landscape of molecular alterations in HCC; however, the most common mutations are not actionable, and only ~25% of tumours harbour potentially targetable drivers. Despite the fact that surveillance programmes lead to early diagnosis in 40-50% of patients, at a point when potentially curative treatments are applicable, almost half of all patients with HCC ultimately receive systemic therapies. Sorafenib was the first systemic therapy approved for patients with advanced-stage HCC, after a landmark study revealed an improvement in median overall survival from 8 to 11 months. New drugs - lenvatinib in the frontline and regorafenib, cabozantinib, and ramucirumab in the second line - have also been demonstrated to improve clinical outcomes, although the median overall survival remains ~1 year; thus, therapeutic breakthroughs are still needed. Immune-checkpoint inhibitors are now being incorporated into the HCC treatment armamentarium and combinations of molecularly targeted therapies with immunotherapies are emerging as tools to boost the immune response. Research on biomarkers of a response or primary resistance to immunotherapies is also advancing. Herein, we summarize the molecular targets and therapies for the management of HCC and discuss the advancements expected in the near future, including biomarker-driven treatments and immunotherapies.
Conflict of interest statement
Competing interests
J.M.L. is a consultant to Bayer HealthCare, Bristol-Myers Squibb (BMS), Celsion, Eisai, Eli Lilly, Exelixis, and Ipsen and has active research funding from Bayer HealthCare, BMS, and Eisai. R.S.F. is a consultant to Bayer HealthCare, BMS, Eisai, Eli Lilly, Merck, Pfizer, and Roche. R.M. and D.S. declare no competing interests.
Figures




References
-
- Torre LA et al. Global cancer statistics, 2012. CA. Cancer J. Clin 65, 87–108 (2015). - PubMed
-
- Llovet JM et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim 2, 16018 (2016). - PubMed
-
- Zucman-Rossi J, Villanueva A, Nault JC & Llovet JM Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149, 1226–1239 (2015). - PubMed
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol 10.1016/j.jhep.2018.03.019 (2018). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

