Nanotherapeutics for Treatment of Pulmonary Arterial Hypertension
- PMID: 30061840
- PMCID: PMC6055049
- DOI: 10.3389/fphys.2018.00890
Nanotherapeutics for Treatment of Pulmonary Arterial Hypertension
Abstract
Pulmonary arterial hypertension (PAH) is a devastating and fatal chronic lung disease. While current pharmacotherapies have improved patient quality of life, PAH drugs suffer from limitations in the form of short-term pharmacokinetics, instability, and poor organ specificity. Traditionally, nanotechnology-based delivery strategies have proven advantageous at increasing both circulation lifetimes of chemotherapeutics and accumulation in tumors due to enhanced permeability through fenestrated vasculature. Importantly, increased nanoparticle (NP) accumulation in diseased tissues has been observed pre-clinically in pathologies characterized by endothelial dysfunction and remodeled vasculature, including myocardial infarction and heart failure. Recently, this phenomenon has also been observed in preclinical models of PAH, leading to the exploration of NP-based drug delivery as a therapeutic modality in PAH. Herein, we discussed the advantages of NPs for efficacious treatment of PAH, including heightened therapeutic delivery to diseased lungs for increased drug bioavailability, as well as highlighted innovative nanotherapeutic approaches for PAH.
Keywords: chronic lung disease; drug delivery; nanomedicine; nanoparticles; pulmonary arterial hypertension.
Figures


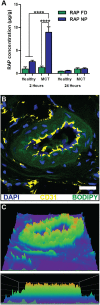
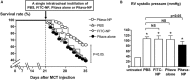
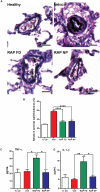

References
-
- Akagi S., Nakamura K., Matsubara H., Kondo M., Miura D., Matoba T., et al. (2016). Intratracheal administration of prostacyclin analogue-incorporated nanoparticles ameliorates the development of monocrotaline and sugen-hypoxia-induced pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 67 290–298. 10.1097/FJC.0000000000000352 - DOI - PMC - PubMed
-
- Anderson R., Franch A., Castell M., Perez-Cano F. J., Brauer R., Pohlers D., et al. (2010). Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis Res. Ther. 12:R147. 10.1186/ar3089 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

