Dynamical Patterning Modules, Biogeneric Materials, and the Evolution of Multicellular Plants
- PMID: 30061903
- PMCID: PMC6055014
- DOI: 10.3389/fpls.2018.00871
Dynamical Patterning Modules, Biogeneric Materials, and the Evolution of Multicellular Plants
Abstract
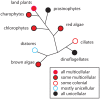
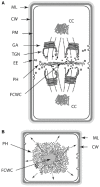
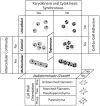
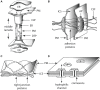
Comparative analyses of developmental processes across a broad spectrum of organisms are required to fully understand the mechanisms responsible for the major evolutionary transitions among eukaryotic photosynthetic lineages (defined here as the polyphyletic algae and the monophyletic land plants). The concepts of dynamical patterning modules (DPMs) and biogeneric materials provide a framework for studying developmental processes in the context of such comparative analyses. In the context of multicellularity, DPMs are defined as sets of conserved gene products and molecular networks, in conjunction with the physical morphogenetic and patterning processes they mobilize. A biogeneric material is defined as mesoscale matter with predictable morphogenetic capabilities that arise from complex cellular conglomerates. Using these concepts, we outline some of the main events and transitions in plant evolution, and describe the DPMs and biogeneric properties associated with and responsible for these transitions. We identify four primary DPMs that played critical roles in the evolution of multicellularity (i.e., the DPMs responsible for cell-to-cell adhesion, identifying the future cell wall, cell differentiation, and cell polarity). Three important conclusions emerge from a broad phyletic comparison: (1) DPMs have been achieved in different ways, even within the same clade (e.g., phycoplastic cell division in the Chlorophyta and phragmoplastic cell division in the Streptophyta), (2) DPMs had their origins in the co-option of molecular species present in the unicellular ancestors of multicellular plants, and (3) symplastic transport mediated by intercellular connections, particularly plasmodesmata, was critical for the evolution of complex multicellularity in plants.
Keywords: algal evolution; convergent evolution; dynamical patterning modules; plant evolution; plasmodesmata.
Figures


References
-
- Beaumont N. (2009). Modelling the transport of nutrients in early animals. Evol. Biol. 36 256–266. 10.1007/s11692-008-9047-2 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

