Application of nSMOL coupled with LC-MS bioanalysis for monitoring the Fc-fusion biopharmaceuticals Etanercept and Abatacept in human serum
- PMID: 30062014
- PMCID: PMC6056752
- DOI: 10.1002/prp2.422
Application of nSMOL coupled with LC-MS bioanalysis for monitoring the Fc-fusion biopharmaceuticals Etanercept and Abatacept in human serum
Abstract
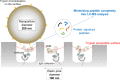
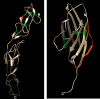
The principle of nano-surface and molecular-orientation limited (nSMOL) proteolysis has a unique characteristic Fab-selective proteolysis for antibody bioanalysis that is independent of a variety of monoclonal antibodies by the binding antibody Fc via Protein A/G in a pore with 100 nm diameter and modified trypsin immobilization on the surface of nanoparticles with 200 nm diameter. Since minimizing peptide complexity and protease contamination while maintaining antibody sequence specificity enables a rapid and broad development of optimized methods for liquid chromatography-mass spectrometry (LC-MS) bioanalysis, the application of regulatory LC-MS for monitoring antibody biopharmaceuticals is expected. nSMOL is theoretically anticipated to be applicable for representative Fc-fusion biopharmaceuticals, because Protein A/G-binding site Fc exists on the C-terminus, and its functional domain is available to orient and interact with the reaction solution. In this report, we describe the validated LC-MS bioanalysis for monitoring Ethanercept and Abatacept using nSMOL technology. The quantitation range of Ethanercept in human serum was from 0.195 to 100 μg/mL using the signature peptide VFCTK (aa.43-47), and that of Abatacept was from 0.391 to 100 μg/mL using the signature peptide MHVAQPAVVLASSR (aa.1-14). Both proteins fulfilled the guideline criteria for low-molecular-weight drug compounds. The results indicate that the clinical and therapeutic monitoring for antibody and Fc-fusion biopharmaceuticals are adequately applicable using nSMOL proteolysis coupled with LC-MS bioanalysis.
Keywords: Abatacept; Etanercept; LC‐MS; bioanalysis; clinical pharmacokinetics; nano‐surface and molecular‐orientation limited proteolysis; therapeutic drug monitoring.
Figures

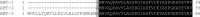
Similar articles
-
Multiplexed monitoring of therapeutic antibodies for inflammatory diseases using Fab-selective proteolysis nSMOL coupled with LC-MS.J Immunol Methods. 2019 Sep;472:44-54. doi: 10.1016/j.jim.2019.06.014. Epub 2019 Jun 12. J Immunol Methods. 2019. PMID: 31201793
-
Regulated LC-MS/MS bioanalysis technology for therapeutic antibodies and Fc-fusion proteins using structure-indicated approach.Drug Metab Pharmacokinet. 2019 Feb;34(1):19-24. doi: 10.1016/j.dmpk.2018.10.002. Epub 2018 Oct 19. Drug Metab Pharmacokinet. 2019. PMID: 30392772 Review.
-
Application of nano-surface and molecular-orientation limited proteolysis to LC-MS bioanalysis of cetuximab.Bioanalysis. 2016 May;8(10):1009-20. doi: 10.4155/bio-2016-0018. Epub 2016 Mar 14. Bioanalysis. 2016. PMID: 26972866
-
Verification between Original and Biosimilar Therapeutic Antibody Infliximab Using nSMOL Coupled LC-MS Bioanalysis in Human Serum.Curr Pharm Biotechnol. 2018;19(6):495-505. doi: 10.2174/1389201019666180703093517. Curr Pharm Biotechnol. 2018. PMID: 29968534 Free PMC article.
-
Internal standards in the quantitative determination of protein biopharmaceuticals using liquid chromatography coupled to mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Apr 15;893-894:1-14. doi: 10.1016/j.jchromb.2012.02.021. Epub 2012 Feb 21. J Chromatogr B Analyt Technol Biomed Life Sci. 2012. PMID: 22426285 Review.
Cited by
-
Low-Intensity Pulsed Ultrasound-Mediated Blood-Brain Barrier Opening Increases Anti-Programmed Death-Ligand 1 Delivery and Efficacy in Gl261 Mouse Model.Pharmaceutics. 2023 Jan 30;15(2):455. doi: 10.3390/pharmaceutics15020455. Pharmaceutics. 2023. PMID: 36839777 Free PMC article.
-
Bioanalysis in the Age of New Drug Modalities.AAPS J. 2021 May 3;23(3):64. doi: 10.1208/s12248-021-00594-w. AAPS J. 2021. PMID: 33942188 Free PMC article. Review.
-
Comparative Pharmacokinetic Analysis of Aflibercept and Brolucizumab in Human Aqueous Humor Using Nano-Surface and Molecular-Orientation Limited Proteolysis.Int J Mol Sci. 2025 Jan 10;26(2):556. doi: 10.3390/ijms26020556. Int J Mol Sci. 2025. PMID: 39859273 Free PMC article.
-
ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates.Antibodies (Basel). 2018 Nov 30;7(4):41. doi: 10.3390/antib7040041. Antibodies (Basel). 2018. PMID: 31544891 Free PMC article. Review.
-
N-terminus of Etanercept is Proteolytically Processed by Dipeptidyl Peptidase-4.Pharm Res. 2022 Oct;39(10):2541-2554. doi: 10.1007/s11095-022-03371-2. Epub 2022 Aug 19. Pharm Res. 2022. PMID: 35986123
References
-
- Suffredini AF, Reda D, Banks SM, Tropea M, Agosti JM, Miller R. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol. 1995;155:5038‐5045. - PubMed
-
- Epstein WV. Treatment of rheumatoid arthritis with a tumor necrosis factor receptor‐Fc fusion protein. N Engl J Med. 1997;337:1559‐1560; author reply 60‐1. - PubMed
-
- Favalli EG, Pontikaki I, Becciolini A, et al. Real‐life 10‐year retention rate of first‐line anti‐TNF drugs for inflammatory arthritides in adult‐ and juvenile‐onset populations: similarities and differences. Clin Rheumatol. 2017;36:1747‐1755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

