Phenotypic screening identifies a new oxazolone inhibitor of necroptosis and neuroinflammation
- PMID: 30062059
- PMCID: PMC6060125
- DOI: 10.1038/s41420-018-0067-0
Phenotypic screening identifies a new oxazolone inhibitor of necroptosis and neuroinflammation
Erratum in
-
Erratum: Publisher Correction: articles initially published in wrong volume.Cell Death Discov. 2019 Jul 10;5:116. doi: 10.1038/s41420-019-0186-2. eCollection 2019. Cell Death Discov. 2019. PMID: 31312525 Free PMC article.
Abstract
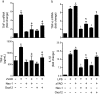
Necroptosis is a regulated form of necrosis, which may be critical in the pathogenesis of neurodegenerative diseases. Neuroinflammation, characterized by the activation of glial cells such as microglia, is closely linked with neurodegenerative pathways and constitutes a major mechanism of neural damage and disease progression. Importantly, inhibition of necroptosis results in disease improvement, unveiling an alternative approach for therapeutic intervention. In the present study, we screened a small library of new molecules, potentially inhibitors of necroptosis, using two cellular models of necroptosis. A new oxazolone, Oxa12, reduced tumour necrosis factor α (TNF-α)-induced necroptosis in mouse L929 fibrosarcoma cells. Notably, Oxa12 strongly inhibited zVAD-fmk-induced necroptosis in murine BV2 microglial cells. Moreover, Oxa12 blocked phosphorylation of mixed-lineage kinase domain-like protein (MLKL), and interfered with necrosome complex formation, indicating that Oxa12 targets components upstream of MLKL. In fact, in silico molecular docking studies revealed that Oxa12 is occupying a region similar to the 1-aminoisoquinoline type II kinase inhibitor inside the receptor-interacting protein 1 (RIP1) kinase domain. Finally, in microglial cells, Oxa12 attenuated zVAD-fmk- and lipopolysaccharide (LPS)-induced inflammatory processes, as revealed by a marked decrease of TNF-α and/or IL-1β expression. More specifically, Oxa12 negatively targeted c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways, as well as NF-κB activation. Overall, we identified a strong lead inhibitor of necroptosis that is also effective at reducing inflammation-associated events. Oxa12 is a promising candidate molecule for further development to target disease states dependent on RIP kinase activity.
Conflict of interest statement
The authors declare that they have no conflict of interest.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

