Role of RAC3 coactivator in the adipocyte differentiation
- PMID: 30062065
- PMCID: PMC6062518
- DOI: 10.1038/s41420-018-0085-y
Role of RAC3 coactivator in the adipocyte differentiation
Erratum in
-
Erratum: Publisher Correction: articles initially published in wrong volume.Cell Death Discov. 2019 Jul 10;5:116. doi: 10.1038/s41420-019-0186-2. eCollection 2019. Cell Death Discov. 2019. PMID: 31312525 Free PMC article.
Abstract
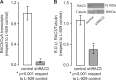
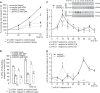
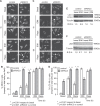
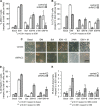
RAC3 is a member of the p160 family of steroid receptor coactivators and it is highly expressed in several human cancers, contributing to enhanced cell proliferation and cellular transformation. In this work, we have studied the role of RAC3 in adipogenesis in L-929 cells. Adipogenesis is a highly regulated process, involving cell cycle arrest and changes in the gene expression pattern required for morphological remodelling. We found that RAC3 expression levels are downregulated during adipocyte differentiation induced by specific stimulus. In addition, cells constitutively expressing low levels of RAC3 (shRNA), showed enhanced adipocyte differentiation which was evidenced by the early detection of the adipocyte markers Perilipin, PPARγ and Oil Red O staining. Moreover, RAC3 downregulation favoured cell arrest and autophagy. Early and late autophagy inhibitors blocked adipocyte differentiation in control cells, but partially inhibited shRAC3 differentiation, demonstrating that although autophagy is required for adipogenesis, additional signals could be trigged by RAC3 downregulation. We conclude that RAC3 is a key regulator of adipogenesis, since its downregulation generates the cellular arrest and autophagic responses that are required steps for this process.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

