Exploring a naturally tailored small molecule for stretchable, self-healing, and adhesive supramolecular polymers
- PMID: 30062126
- PMCID: PMC6063538
- DOI: 10.1126/sciadv.aat8192
Exploring a naturally tailored small molecule for stretchable, self-healing, and adhesive supramolecular polymers
Abstract
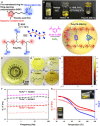
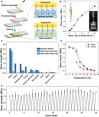
Polymeric materials with integrated functionalities are required to match their ever-expanding practical applications, but there is always a trade-off between complex material performances and synthetic simplification. A simple and effective synthesis route is reported to transform a small molecule of biological origin, thioctic acid, into a high-performance supramolecular polymeric material, which combines processability, ultrahigh stretchability, rapid self-healing ability, and reusable adhesivity to surfaces. The proposed one-step preparation process of this material involves the mixing of three commercially available feedstocks at mild temperature without any external solvent and a subsequent cooling process that resulted in a dynamic, high-density, and dry supramolecular polymeric network cross-linked by three different types of dynamic chemical bonds, whose cooperative effects in the network enable high performance of this supramolecular polymeric material.
Figures


References
-
- Lutz J.-F., Lehn J.-M., Meijer E. W., Matyjaszewski K., From precision polymers to complex materials and systems. Nat. Rev. Mater. 1, 16024 (2016).
-
- Wojtecki R. J., Meador M. A., Rowan S. J., Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 10, 14–27 (2011). - PubMed
-
- Amabilino D. B., Smith D. K., Steed J. W., Supramolecular materials. Chem. Soc. Rev. 46, 2404–2420 (2017). - PubMed
-
- Yang L., Tan X., Wang Z., Zhang X., Supramolecular polymers: Historical development, preparation, characterization, and functions. Chem. Rev. 115, 7196–7239 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

