The secrets of the stability of the HIV-1 capsid
- PMID: 30063007
- PMCID: PMC6067877
- DOI: 10.7554/eLife.38895
The secrets of the stability of the HIV-1 capsid
Abstract
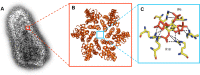
Structural and biophysical studies help to follow the disassembly of the HIV-1 capsid in vitro, and reveal the role of a small molecule called IP6 in regulating capsid stability.
Keywords: CA lattice; HIV-1; IP6; PF74; R18 pore; capsid; human; infectious disease; microbiology; molecular biophysics; structural biology; virus.
© 2018, Obr et al.
Conflict of interest statement
MO, HK No competing interests declared
Figures
Comment on
-
IP6 is an HIV pocket factor that prevents capsid collapse and promotes DNA synthesis.Elife. 2018 May 31;7:e35335. doi: 10.7554/eLife.35335. Elife. 2018. PMID: 29848441 Free PMC article.
-
Kinetics of HIV-1 capsid uncoating revealed by single-molecule analysis.Elife. 2018 Jun 7;7:e34772. doi: 10.7554/eLife.34772. Elife. 2018. PMID: 29877795 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

