Delayed onset of neurosarcoidosis after concurrent ipilimumab/nivolumab therapy
- PMID: 30064495
- PMCID: PMC6069826
- DOI: 10.1186/s40425-018-0390-2
Delayed onset of neurosarcoidosis after concurrent ipilimumab/nivolumab therapy
Abstract
Background: Immune checkpoint inhibitors have transformed the treatment landscape for many cancers, including metastatic melanoma, but have also opened the door for a diverse variety of immune-related adverse effects.
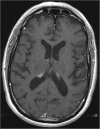
Case presentation: We describe the first reported case of presumed neurosarcoidosis as an immune-related adverse effect that developed nearly a year after discontinuation of treatment with combination ipilimumab and nivolumab for recurrent metastatic melanoma. The patient was noted to develop clinical signs consistent with systemic sarcoidosis shortly after the initiation of treatment and underwent a biopsy of hilar lymphadenopathy that confirmed sarcoidosis and after which immunotherapy was discontinued. His melanoma remained stable on surveillance imaging for the next year after which time he developed neurological symptoms and was found to have MRI brain abnormalities without evidence of intracranial metastatic disease, consistent with probable neurosarcoidosis given biopsy-proven systemic sarcoidosis and lack of evidence of CNS infection or malignancy. He underwent treatment with high dose steroids, followed by infliximab, and then methotrexate with both clinical and radiographic improvement within 4 months of starting treatment.
Conclusions: Immune-related adverse effects often occur within 3-6 months of receiving immune checkpoint inhibitor therapy, with some reports of late toxicity. This report highlights a case of probable neurosarcoidosis nearly a year after discontinuation of immune checkpoint therapy. The potential for durable responses after discontinuation of therapy also likely underscores a potential for late toxicity. In patients presenting with new or unexplained symptoms after checkpoint inhibitor therapy, the index of suspicion for an immune-related adverse effect should remain high, irrespective of timing.
Keywords: Immune-related adverse events; Ipilimumab; Neurosarcoidosis; Nivolumab.
Conflict of interest statement
Ethics approval and consent to participate
No formal ethics approval was needed since we were reporting an observation case report. Consent was obtained from the patient.
Consent for publication
Consent was obtained from the patient. He signed a generic consent for this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Larkin J, Chiarion-Silen V, Gonzalez R, et al. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (CheckMate 067) Washington, DC: Oral presentation presented at, The American Association of Cancer Research (AACR) Annual Meeting; 2017.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
