Assessment of the effect of addition of 24 hours of oral tranexamic acid post-operatively to a single intraoperative intravenous dose of tranexamic acid on calculated blood loss following primary hip and knee arthroplasty (TRAC-24): a study protocol for a randomised controlled trial
- PMID: 30064517
- PMCID: PMC6069723
- DOI: 10.1186/s13063-018-2784-3
Assessment of the effect of addition of 24 hours of oral tranexamic acid post-operatively to a single intraoperative intravenous dose of tranexamic acid on calculated blood loss following primary hip and knee arthroplasty (TRAC-24): a study protocol for a randomised controlled trial
Abstract
Background: While it is has been proven that tranexamic acid (TXA) reduces blood loss in primary total hip and knee arthroplasty (THA and TKA), there is little published evidence on the use of TXA beyond 3 h post-operatively. Most blood loss occurs after wound closure and the primary aim of this study is to determine if the use of oral TXA post-operatively for up to 24 h will reduce calculated blood loss at 48 h beyond an intra-operative intravenous bolus alone following primary THA and TKA. To date, most TXA studies have excluded patients with a history of thromboembolic disease.
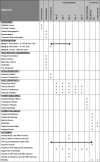
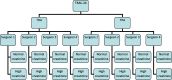
Methods/design: This is a phase IV, single-centred, open-label, parallel-group, randomised controlled trial. Participants are randomised to one of three groups: group 1, an intravenous (IV) bolus of TXA peri-operatively plus oral TXA post-operatively for 24 h; group 2, an IV bolus of TXA peri-operatively or group 3, standard care (no TXA). Eligible participants, including those with a history of thromboembolic disease, are allocated to these groups with a 2:2:1 allocation ratio. The primary outcome is the indirectly calculated blood loss 48 h after surgery. Researchers and patients are not blinded to the treatment; however, staff processing blood samples are. Originally 1166 participants were required to complete this study, 583 THA and 583 TKA. However, following an interim analysis after 100 THA and 100 TKA participants had been recruited to the study, the data monitoring ethics committee recommended stopping group 3 (standard care).
Discussion: TRAC-24 will help to determine whether an extended TXA dosing regimen can further reduce blood loss following primary THA and TKA. By including patients with a history of thromboembolic disease, this study will add to our understanding of the safety profile of TXA in this clinical situation.
Trial registration: ISRCTN registry, ISRCTN58790500 . Registered on 3 June 2016, EudraCT: 2015-002661-36.
Keywords: Arthroplasty; Blood loss; Hip; Knee; Oral; Post-operative; Replacement; Tranexamic acid.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was granted for this clinical trial by East of England – Cambridge East Research Ethics Committee (16/EE/0068). Clinical trial authorisation has been granted by the MHRA ( 32485/0030/001–0001). All participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

