Bacterial zinc uptake regulator proteins and their regulons
- PMID: 30065104
- PMCID: PMC6103462
- DOI: 10.1042/BST20170228
Bacterial zinc uptake regulator proteins and their regulons
Abstract
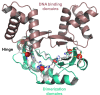
All organisms must regulate the cellular uptake, efflux, and intracellular trafficking of essential elements, including d-block metal ions. In bacteria, such regulation is achieved by the action of metal-responsive transcriptional regulators. Among several families of zinc-responsive transcription factors, the 'zinc uptake regulator' Zur is the most widespread. Zur normally represses transcription in its zinc-bound form, in which DNA-binding affinity is enhanced allosterically. Experimental and bioinformatic searches for Zur-regulated genes have revealed that in many cases, Zur proteins govern zinc homeostasis in a much more profound way than merely through the expression of uptake systems. Zur regulons also comprise biosynthetic clusters for metallophore synthesis, ribosomal proteins, enzymes, and virulence factors. In recognition of the importance of zinc homeostasis at the host-pathogen interface, studying Zur regulons of pathogenic bacteria is a particularly active current research area.
Keywords: Zur; bacteria; metal ions; zinc uptake regulator; zinc-responsive transcription factors.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures


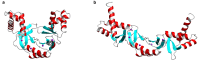


References
-
- Dupont C.L. and Caetano-Anolles G. (2010) Mulkidjanian and Galperin: Zn may have constrained evolution during the Proterozoic but not the archean reply. Proc. Natl Acad. Sci. U.S.A. 107, E138 10.1073/pnas.1009565107 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

