Emerging mechanisms of dynein transport in the cytoplasm versus the cilium
- PMID: 30065109
- PMCID: PMC6103457
- DOI: 10.1042/BST20170568
Emerging mechanisms of dynein transport in the cytoplasm versus the cilium
Abstract
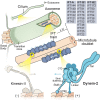
Two classes of dynein power long-distance cargo transport in different cellular contexts. Cytoplasmic dynein-1 is responsible for the majority of transport toward microtubule minus ends in the cell interior. Dynein-2, also known as intraflagellar transport dynein, moves cargoes along the axoneme of eukaryotic cilia and flagella. Both dyneins operate as large ATP-driven motor complexes, whose dysfunction is associated with a group of human disorders. But how similar are their mechanisms of action and regulation? To examine this question, this review focuses on recent advances in dynein-1 and -2 research, and probes to what extent the emerging principles of dynein-1 transport could apply to or differ from those of the less well-understood dynein-2 mechanoenzyme.
Keywords: cilia; dynein; intraflagellar transport; kinesin; microtubule.
© 2018 The Author(s).
Conflict of interest statement
Note added in proof
An analysis of dynein-2 subunit interactions via a visible immunoprecipitation (VIP) assay has been published, demonstrating interactions between WDR60 and TCTEX1/3-TCTEX1D2; WDR34 and LC8 and Roadblock; and the intermediate chain-light chain complex with the heavy chain and light-intermediate chain [148].
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

