Dienogest Versus Leuprolide Acetate for Recurrent Pelvic Pain Following Laparoscopic Treatment of Endometriosis
- PMID: 30065547
- PMCID: PMC6046679
- DOI: 10.1007/s13224-018-1119-3
Dienogest Versus Leuprolide Acetate for Recurrent Pelvic Pain Following Laparoscopic Treatment of Endometriosis
Abstract
Objective: To compare the efficacy and safety of dienogest (DNG) with depot leuprolide acetate (LA) in patients with recurrent pelvic pain following laparoscopic surgery for endometriosis.
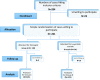
Design: Prospective randomized trial.
Setting: Zagazig University hospitals, Egypt.
Patients: Two hundred and forty-two patients with recurrent pelvic pain following laparoscopic surgery for endometriosis.
Intervention: Dienogest (2 mg/day, orally) or depot LA (3.75 mg/4 weeks, intramuscularly) for 12 weeks.
Main outcome measures: A visual analogue scale was used to test the intensity of pain before and after the end of treatment.
Results: There was highly significant reduction in pelvic pain, back pain and dyspareunia in both groups with mean of difference in dienogest group (28.7 ± 5.3, 19.0 ± 4.3 and 20.0 ± 3.08 mm, respectively) and in LA group (26.2 ± 3.01, 19.5 ± 3.01 and 17.9 ± 2.9 mm, respectively). The most frequent drug-related adverse effects in dienogest group were vaginal bleeding and weight gain (64.5 and 10.8%, respectively) which were significantly higher than LA group (21.5 and 3.3%, respectively). While the most frequent drug-related adverse effects in LA group were hot flushes and vaginal dryness (46.3 and 15.7%, respectively) which were significantly higher than dienogest group (15.7 and 3.3%, respectively).
Conclusion: Daily dienogest is as effective as depot LA for relieving endometriosis-associated pelvic pain, low back pain and dyspareunia. In addition, dienogest has acceptable safety, tolerability and lower incidence of hot flushes. Thus, it may offer an effective and well-tolerated treatment in endometriosis.
Keywords: Dienogest; Endometriosis; Leuprolide acetate; Pelvic pain.
Conflict of interest statement
Compliance with Ethical StandardsAll authors declare that there is no conflict of interest with other people or organizations that could inappropriately influence or bias the content of the paper.All procedures performed in this study involving human participants (administration of oral dienogest 2 mg once daily or intramuscular leuprolide acetate depot 3.75 mg injection every 4 weeks for 12 weeks for patients with recurrent pelvic pain following laparoscopy for endometriosis) were in accordance with the ethical standards of the Faculty of Human Medicine—Zagazig University and with the 1964 Declaration of Helsinki and its later amendments and were approved by the IRB (Institutional Review Board). The study protocol, the intervention involved, possible early and long-term side effects of interventions were explained to the patients. After approval of the local ethics committee, a written informed consent was obtained from all patients before participating in the study.Written informed consent was obtained from all patients before participating in the study.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources