Oxytocin Relieves Neuropathic Pain Through GABA Release and Presynaptic TRPV1 Inhibition in Spinal Cord
- PMID: 30065629
- PMCID: PMC6056657
- DOI: 10.3389/fnmol.2018.00248
Oxytocin Relieves Neuropathic Pain Through GABA Release and Presynaptic TRPV1 Inhibition in Spinal Cord
Abstract
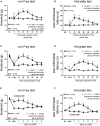
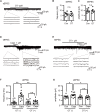
Objective: Oxytocin (OT) is synthesized within the paraventricular nucleus and supraoptic nucleus of the hypothalamus. In addition to its role in uterine contraction, OT plays an important antinociceptive role; however, the underlying molecular mechanisms of antinociceptive role of OT remain elusive. We hypothesized that the antinociceptive effect of OT on neuropathic pain may occur via inhibition of TRPV1 activation in the spinal cord. The present study explores the antinociceptive role of OT and its mechanisms in neuropathic pain. Methods: Partial sciatic nerve ligation (pSNL) was performed to induce neuropathic pain. Animal behaviors were measured using a set of electronic von Frey apparatus and hot plate. Electrophysiological recordings and molecular biological experiments were performed. Results: Intrathecal administration of OT alleviated both mechanical allodynia and thermal hyperalgesia in pSNL rats (n = 6, per group, P < 0.0001, saline vs. OT group). Electrophysiological data revealed that OT significantly inhibited the enhancement of frequency and amplitude of spontaneous excitatory post-synaptic currents induced presynaptically by TRPV1 activation in the spinal cord. Moreover, the inhibitory effect of OT on capsaicin-induced facilitation of excitatory transmission was blocked by co-treatment with saclofen, while intrathecal administration of OT dramatically inhibited capsaicin-induced ongoing pain in rats, (n = 6, per group, P < 0.0001, saline vs. OT group). The paw withdrawal latency in response to heat stimulation was significantly impaired in TRPV1KO mice 3 days after pSNL upon OT (i.t.) treatment, compared with wild type mice (n = 6, P < 0.05). Finally, OT prevented TRPV1 up-regulation in spinal cords of pSNL model rats. Conclusion: OT relieves neuropathic pain through GABA release and presynaptic TRPV1 inhibition in the spinal cord. OT and its receptor system might be an intriguing target for the treatment and prevention of neuropathic pain.
Keywords: GABA; TRPV1; mechanical allodynia; neuropathic pain; oxytocin; spinal cord; thermal hyperalgesia.
Figures






Similar articles
-
Ameliorative potential of standardized fruit extract of Pterodon pubescens Benth on neuropathic pain in mice: Evidence for the mechanisms of action.J Ethnopharmacol. 2015 Dec 4;175:273-86. doi: 10.1016/j.jep.2015.09.005. Epub 2015 Sep 18. J Ethnopharmacol. 2015. PMID: 26386380
-
Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain.Anesthesiology. 2002 May;96(5):1161-7. doi: 10.1097/00000542-200205000-00020. Anesthesiology. 2002. PMID: 11981157
-
A critical time window for the analgesic effect of central histamine in the partial sciatic ligation model of neuropathic pain.J Neuroinflammation. 2016 Jun 24;13(1):163. doi: 10.1186/s12974-016-0637-0. J Neuroinflammation. 2016. PMID: 27342775 Free PMC article.
-
Tumor necrosis factor-mediated downregulation of spinal astrocytic connexin43 leads to increased glutamatergic neurotransmission and neuropathic pain in mice.Brain Behav Immun. 2015 Oct;49:293-310. doi: 10.1016/j.bbi.2015.06.015. Epub 2015 Jun 24. Brain Behav Immun. 2015. PMID: 26116449
-
Implications of intrathecal pertussis toxin animal model on the cellular mechanisms of neuropathic pain syndrome.Acta Anaesthesiol Sin. 2003 Dec;41(4):187-96. Acta Anaesthesiol Sin. 2003. PMID: 14768516 Review.
Cited by
-
Three-Day Continuous Oxytocin Infusion Attenuates Thermal and Mechanical Nociception by Rescuing Neuronal Chloride Homeostasis via Upregulation KCC2 Expression and Function.Front Pharmacol. 2022 Mar 24;13:845018. doi: 10.3389/fphar.2022.845018. eCollection 2022. Front Pharmacol. 2022. PMID: 35401174 Free PMC article.
-
Kampo Formulae for the Treatment of Neuropathic Pain ∼ Especially the Mechanism of Action of Yokukansan ∼.Front Mol Neurosci. 2021 Dec 14;14:705023. doi: 10.3389/fnmol.2021.705023. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34970116 Free PMC article. Review.
-
Neuropeptide therapeutics to repress lateral septum neurons that disable sociability in an autism mouse model.Cell Rep Med. 2024 Nov 19;5(11):101781. doi: 10.1016/j.xcrm.2024.101781. Epub 2024 Oct 17. Cell Rep Med. 2024. PMID: 39423809 Free PMC article.
-
Automated Stereotactic Gamma Ray Radiosurgery to the Pituitary Gland in Terminally Ill Cancer Patients with Opioid Refractory Pain.Cureus. 2019 Jun 3;11(6):e4811. doi: 10.7759/cureus.4811. Cureus. 2019. PMID: 31403008 Free PMC article.
-
A comprehensive guide to MEGA-PRESS for GABA measurement.Anal Biochem. 2023 May 15;669:115113. doi: 10.1016/j.ab.2023.115113. Epub 2023 Mar 21. Anal Biochem. 2023. PMID: 36958511 Free PMC article. Review.
References
-
- Breton J. D., Veinante P., Uhl-Bronner S., Vergnano A. M., Freund-Mercier M. J., Schlichter R., et al. (2008). Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I-II which amplify GABAergic inhibition. Mol. Pain 4:19. 10.1186/1744-8069-4-19 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

