Pericyte Structural Remodeling in Cerebrovascular Health and Homeostasis
- PMID: 30065645
- PMCID: PMC6057109
- DOI: 10.3389/fnagi.2018.00210
Pericyte Structural Remodeling in Cerebrovascular Health and Homeostasis
Abstract
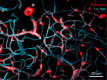
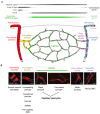
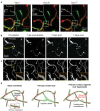
The biology of brain microvascular pericytes is an active area of research and discovery, as their interaction with the endothelium is critical for multiple aspects of cerebrovascular function. There is growing evidence that pericyte loss or dysfunction is involved in the pathogenesis of Alzheimer's disease, vascular dementia, ischemic stroke and brain injury. However, strategies to mitigate or compensate for this loss remain limited. In this review, we highlight a novel finding that pericytes in the adult brain are structurally dynamic in vivo, and actively compensate for loss of endothelial coverage by extending their far-reaching processes to maintain contact with regions of exposed endothelium. Structural remodeling of pericytes may present an opportunity to foster pericyte-endothelial communication in the adult brain and should be explored as a potential means to counteract pericyte loss in dementia and cerebrovascular disease. We discuss the pathophysiological consequences of pericyte loss on capillary function, and the biochemical pathways that may control pericyte remodeling. We also offer guidance for observing pericytes in vivo, such that pericyte structural remodeling can be more broadly studied in mouse models of cerebrovascular disease.
Keywords: Alzheimer’s disease; blood-brain barrier; capillary blood flow; mural cell; neurovascular coupling; pericyte; stroke; two-photon imaging.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

