Bioprotective Effect of Lactococcus piscium CNCM I-4031 Against Listeria monocytogenes Growth and Virulence
- PMID: 30065705
- PMCID: PMC6056605
- DOI: 10.3389/fmicb.2018.01564
Bioprotective Effect of Lactococcus piscium CNCM I-4031 Against Listeria monocytogenes Growth and Virulence
Abstract
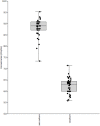
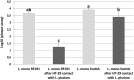
Listeria monocytogenes is a Gram-positive pathogen occurring in many refrigerated ready-to-eat foods. It is responsible for foodborne listeriosis, a rare but severe disease with a high mortality rate (20-30%). Lactococcus piscium CNCM I-4031 has the capacity to prevent the growth of L. monocytogenes in contaminated peeled and cooked shrimp and in a chemically defined medium using a cell-to-cell contact-dependent mechanism. To characterize this inhibition further, the effect of L. piscium was tested on a collection of 42 L. monocytogenes strains. All strains were inhibited but had different sensitivities. The effect of the initial concentration of the protective and the target bacteria revealed that the inhibition always occurred when L. piscium had reached its maximum population density, whatever the initial concentration of the protective bacteria. Viewed by scanning electron microscopy, L. monocytogenes cell shape and surface appeared modified in co-culture with L. piscium CNCM I-4031. Lastly, L. monocytogenes virulence, evaluated by a plaque-forming assay on the HT-29 cell line, was reduced after cell pre-treatment by the protective bacteria. In conclusion, the bioprotective effect of L. piscium toward L. monocytogenes growth and virulence was demonstrated, and a hypothesis for the inhibition mechanism is put forward.
Keywords: Lactococcus piscium; Listeria monocytogenes; biopreservation; cell ratio; co-culture; scanning electron microscopy; virulence.
Figures
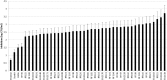
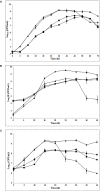
 ) in pure culture (solid line) and in co-culture (dotted line) in MSMA at 26°C. Initial concentrations of L. piscium/L. monocytogenes: (A) 103/103 CFU/ml, (B) 105/103 CFU/ml, and (C) 106/106 CFU/ml.
) in pure culture (solid line) and in co-culture (dotted line) in MSMA at 26°C. Initial concentrations of L. piscium/L. monocytogenes: (A) 103/103 CFU/ml, (B) 105/103 CFU/ml, and (C) 106/106 CFU/ml.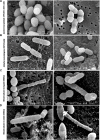
References
-
- Batdorj B., Trinetta V., Dalgalarrondo M., Prevost H., Dousset X., Ivanova I., et al. (2007). Isolation, taxonomic identification and hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis T 31, isolated from Mongolian yoghurt: inhibitory activity on food-borne pathogens. J. Appl. Microbiol. 103 584–593. 10.1111/j.1365-2672.2007.03279.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

