Breast cancer biomarkers in clinical testing: analysis of a UK national external quality assessment scheme for immunocytochemistry and in situ hybridisation database containing results from 199 300 patients
- PMID: 30066480
- PMCID: PMC6174620
- DOI: 10.1002/cjp2.112
Breast cancer biomarkers in clinical testing: analysis of a UK national external quality assessment scheme for immunocytochemistry and in situ hybridisation database containing results from 199 300 patients
Erratum in
-
'Breast cancer biomarkers in clinical testing: analysis of a UK NEQAS ICC & ISH database containing results from 199 300 patients': by Andrew Dodson1,2 , Suzanne Parry3 , Merdol Ibrahim3 , John MS Bartlett4 , Sarah Pinder5 , Mitch Dowsett1,2 and Keith Miller3 , J Pathol Clin Res 2018; 4: 262-273, https://doi.org/10.1002/cjp2.112.J Pathol Clin Res. 2020 Jul;6(3):227. doi: 10.1002/cjp2.173. J Pathol Clin Res. 2020. PMID: 32633474 Free PMC article. No abstract available.
Abstract
We describe a collated data set of results from clinical testing of breast cancers carried out between 2009 and 2016 in the United Kingdom and Republic of Ireland. More than 199 000 patient biomarker data sets, together with clinicopathological parameters were collected. Our analyses focused on human epidermal growth factor receptor-2 (HER2), oestrogen receptor (ER) and progesterone receptor (PR), with the aim of the study being to provide robust confirmatory evidence on known associations in these biomarkers and to uncover new data on previously undescribed or unconfirmed associations, thus strengthening the evidence-base in clinical breast cancer testing. Overall, 13.1% of tumours were HER2-positive; 10.6% in ER-positive tumours, and 25.5% in ER-negative tumours. Higher rates of HER2 positivity were significantly associated with patient age <56 years versus age ≥56 years, symptomatic versus screen-detected tumours, testing of involved axillary node versus primary breast cancer, invasive ductal carcinoma (not otherwise specified) versus other histological types, higher histological grade, increasing tumour size, increasing nodal involvement, ER-negative versus ER-positive tumour status, PR-negative versus PR-positive tumour status. Where ER status was known, 82.7% of tumours were ER-positive; 80.9% in women age <56 years, and 83.6% in those age ≥56 years (ER-positive cut-off ≥1.0% positive tumour cells or equivalent). Where PR status was known, 64.9% of tumours were PR-positive; 65.8% in women age <56 years, and 64.4% in women age ≥56 years (PR-positive cut off ≥10.0% or equivalent). These analyses of clinical test results provide contemporary benchmarking data for HER2, ER and PR positive rates.
Keywords: ER; HER2; PR; biomarkers; breast cancer; clinical testing; external quality assessment; human epidermal growth factor receptor-2; oestrogen receptor; progesterone receptor.
© 2018 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures
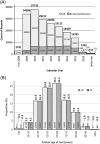

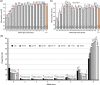
References
-
- UK‐NEQAS ICC & ISH . [Accessed 01 May 2018]. Available from: http://www.ukneqasiccish.org
-
- Ibrahim M, Herriot K, Parry S, et al Audit of breast biomarkers markers, HER2.ER & PR in the UK: an update by the UK National External Quality Assessment Scheme for Immunocytochemistry and In Situ Hybridisation (UK NEQAS ICC & ISH) Virchows Archiv 2011; 459: S74.
-
- Bartlett JM, Ellis IO, Dowsett M, et al Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor (ER) negative, but with grade in those with ER‐positive early‐stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol 2007; 25: 4423–4430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

