Emerging strategies for combination checkpoint modulators in cancer immunotherapy
- PMID: 30067248
- PMCID: PMC6063475
- DOI: 10.1172/JCI120775
Emerging strategies for combination checkpoint modulators in cancer immunotherapy
Abstract
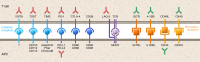
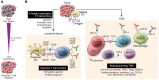
Current immune checkpoint-modulating agents have demonstrated clinical efficacy in certain tumor types, particularly those with a high burden of tumor-specific neoantigens, high tumor-mutational burden, and abundant tumor-infiltrating T cells. However, these tumors often stop responding, with signs of T cells exhaustion, decreased T cell effector function, and upregulated inhibitory checkpoints. To enhance antitumor immunity and rescue exhausted T cells, newer inhibitory and stimulatory checkpoint modulators are being tested as monotherapy or in combination with approved checkpoint inhibitors. In contrast, tumors with low tumor-mutational burden, low neoantigen burden, and a paucity of T cells are immunologically "cold," and therefore first require the addition of agents to facilitate the induction of T cells into tumors. Cold tumors also often recruit immunosuppressive cell subsets, including regulatory T cells, myeloid-derived suppressor cells, and macrophages, and secrete immunosuppressive soluble cytokines, chemokines, and metabolites. To unleash an optimal antitumor immune response, combinatorial therapeutics that combine immune checkpoints with other modalities, such as vaccines, are being developed. From current preclinical data, it appears that combinatorial strategies will provide robust and durable responses in patients with immunologically cold cancers.
Conflict of interest statement
Figures

References
-
- Burnet FM. Immunological surveillance in neoplasia. Transplant Rev. 1971;7:3–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

