Geospatial distribution of Mycobacterium tuberculosis genotypes in Africa
- PMID: 30067763
- PMCID: PMC6070189
- DOI: 10.1371/journal.pone.0200632
Geospatial distribution of Mycobacterium tuberculosis genotypes in Africa
Abstract
Objective: To investigate the distribution of Mycobacterium tuberculosis genotypes across Africa.
Methods: The SITVIT2 global repository and PUBMED were searched for spoligotype and published genotype data respectively, of M. tuberculosis from Africa. M. tuberculosis lineages in Africa were described and compared across regions and with those from 7 European and 6 South-Asian countries. Further analysis of the major lineages and sub-lineages using Principal Component analysis (PCA) and hierarchical cluster analysis were done to describe clustering by geographical regions. Evolutionary relationships were assessed using phylogenetic tree analysis.
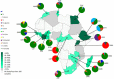
Results: A total of 14727 isolates from 35 African countries were included in the analysis and of these 13607 were assigned to one of 10 major lineages, whilst 1120 were unknown. There were differences in geographical distribution of major lineages and their sub-lineages with regional clustering. Southern African countries were grouped based on high prevalence of LAM11-ZWE strains; strains which have an origin in Portugal. The grouping of North African countries was due to the high percentage of LAM9 strains, which have an origin in the Eastern Mediterranean region. East African countries were grouped based on Central Asian (CAS) and East-African Indian (EAI) strain lineage possibly reflecting historic sea trade with Asia, while West African Countries were grouped based on Cameroon lineage of unknown origin. A high percentage of the Haarlem lineage isolates were observed in the Central African Republic, Guinea, Gambia and Tunisia, however, a mixed distribution prevented close clustering.
Conclusions: This study highlighted that the TB epidemic in Africa is driven by regional epidemics characterized by genetically distinct lineages of M. tuberculosis. M. tuberculosis in these regions may have been introduced from either Europe or Asia and has spread through pastoralism, mining and war. The vast array of genotypes and their associated phenotypes should be considered when designing future vaccines, diagnostics and anti-TB drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Streicher EM, Warren RM, Kewley C, Simpson J, Rastogi N, Sola C, et al. Genotypic and phenotypic characterization of drug-resistant Mycobacterium tuberculosis isolates from rural districts of the Western Cape Province of South Africa. J Clin Microbiol. 2004; 42: 891–894. 10.1128/JCM.42.2.891-894.2004 - DOI - PMC - PubMed
-
- Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006; 6: 23 10.1186/1471-2180-6-23 - DOI - PMC - PubMed
-
- Filliol I, Driscoll JR, van Soolingen D, Kreiswirth BN, Kremer K, Valétudie G, et al. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J Clin Microbiol. 2003; 41: 1963–1970. 10.1128/JCM.41.5.1963-1970.2003 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

