Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews
- PMID: 30067867
- PMCID: PMC6513377
- DOI: 10.1002/14651858.CD012082.pub2
Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews
Abstract
Background: Globally, sickle cell disease (SCD) is one of the commonest severe monogenic disorders, due to the inheritance of two abnormal haemoglobin (beta globin) genes. SCD can cause severe pain, significant end-organ damage, pulmonary complications, and premature death. Red blood cell (RBC) transfusions are used to treat complications of SCD, e.g. acute chest syndrome (ACS) (this often involves a single transfusion episode), or they can be part of a regular long-term transfusion programme to prevent SCD complications.
Objectives: To summarize the evidence in Cochrane Reviews of the effectiveness and safety of RBC transfusions versus no transfusion, or restrictive (to increase the total haemoglobin) versus liberal (to decrease the haemoglobin S level below a specified percentage) transfusion, for treating or preventing complications experienced by people with SCD.
Methods: We included Cochrane Reviews of randomised or quasi-randomised controlled trials published in the Cochrane Database of Systematic Reviews, that addressed various SCD complications and had RBC transfusion as an intervention or comparator. We assessed the methodological quality of included reviews according to the AMSTAR quality assessment.
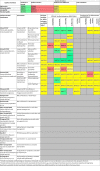
Main results: We included 15 Cochrane Reviews, 10 of which had no included studies with an RBC transfusion intervention (five reported RCTs with other interventions; and five contained no studies). Five of the 15 reviews included participants randomised to RBC transfusion, but in one of these reviews only 10 participants were randomised with no usable data. Four reviews (nine trials with 1502 participants) reported data comparing short- or long-term RBC transfusions versus standard care, disease-modifying agents, a restrictive versus a liberal transfusion strategy and long-term RBC transfusions versus transfusions to treat complications. All reviews were of high quality according to AMSTAR quality assessment, however, the quality of the included trials was highly variable across outcomes. Trials were downgraded according to GRADE methodology for risk of bias, indirectness (most trials were conducted in children with HbSS), and imprecision (outcomes had wide confidence intervals).In all four reviews and all comparisons there was little or no difference in the risk of death (very low-quality evidence). There were either no deaths or death was a rare event.Short-term RBC transfusion versus standard care (one review: two trials, 434 participants, GRADE very low- to low-quality evidence)In people undergoing low- to medium-risk surgery, RBC transfusions may decrease the risk of acute chest syndrome (ACS) in people with African haplotypes compared to standard care (low-quality evidence), but there was little or no difference in people with the Arabic haplotype (very-low quality evidence). There was also little or no difference in the risk of other SCD-related or transfusion-related complications (very-low quality evidence).Long-term RBC transfusion versus standard care (two reviews: three trials, 405 participants, very low- to moderate-quality evidence)In children and adolescents at high risk of stroke (abnormal transcranial doppler (TCD) velocities or silent cerebral infarct (SCI)), long-term RBC transfusions probably decrease the risk of stroke (moderate-quality evidence) and may decrease the risk of ACS and painful crisis compared to standard care (low-quality evidence). Long-term RBC transfusions may also decrease the risk of SCI in children with abnormal TCD velocities (low-quality evidence), but there may be little or no difference in the risk of SCI in children with normal TCD velocities and previous SCI (low-quality evidence).In children and adolescents already receiving long-term RBC transfusions for preventing stroke, in comparison to standard care, continuing long-term RBC transfusions may reduce the risk of SCI (low-quality evidence) but we do not know whether there is a difference in the risk of stroke (very-low quality evidence). In children with normal TCD velocities and SCI there was little or no difference in the risk of alloimmunisation or transfusion reactions, but RBC transfusions may increase the risk of iron overload (low-quality evidence).Long-term RBC transfusion versus RBC transfusion to treat complications (one review: one trial, 72 participants, very low- to low-quality evidence)In pregnant women, long-term RBC transfusions may decrease the risk of painful crisis compared to transfusion for complications (low-quality evidence); but there may be little or no difference in the risk of other SCD-related complications or transfusion reactions (very-low quality evidence).RBC transfusion versus disease-modifying agents (hydroxyurea) (two reviews: two trials; 254 participants, very low- to low-quality evidence)For primary prevention of stroke in children, with abnormal TCD and no severe vasculopathy on magnetic resonance imaging/magnetic resonance angiography (MRI/MRA), who have received at least one year of RBC transfusions, we do not know whether there is a difference between RBC transfusion and disease-modifying agents in the risk of stroke; SCI; ACS; or painful crisis (very-low quality evidence). There may be little or no difference in the risk of iron overload (low-quality evidence).Similarly, for secondary prevention of stroke in children and adolescents, we do not know whether there is a difference between these interventions in the risk of stroke; SCI; or ACS (very-low quality evidence); but hydroxyurea with phlebotomy may increase the risk of painful crisis and global SCD serious adverse events compared to RBC transfusion (low-quality evidence). There may be little or no difference in the risk of iron overload (low-quality evidence).Restrictive versus liberal RBC transfusion strategy (one review: one trial; 230 participants, very low-quality evidence)In people undergoing cholecystectomy, there was little or no difference between strategies in the risk of SCD-related or transfusion-related complications (very-low quality evidence).
Authors' conclusions: This overview provides support from two high-quality Cochrane Reviews for the use of RBC transfusions in preventing stroke in children and adolescents at high risk of stroke (abnormal TCDs or SCI) and evidence that it may decrease the risk of SCI in children with abnormal TCD velocities. In addition RBC transfusions may reduce the risk of ACS and painful crisis in this population.This overview highlights the lack of high-quality evidence in adults with SCD and the number of reviews that have no evidence for the use of RBC transfusions across a spectrum of SCD complications. Also of concern is the variable and often incomplete reporting of patient-relevant outcomes in the included trials such as SCD-related serious adverse events and quality of life.
Conflict of interest statement
Patricia Fortin: funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Lise Estcourt: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Update of
-
Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews.Cochrane Database Syst Rev. 2016 Feb 8;2016(2):CD012082. doi: 10.1002/14651858.CD012082. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Aug 01;8:CD012082. doi: 10.1002/14651858.CD012082.pub2. PMID: 27069421 Free PMC article. Updated.
References
References to included reviews
Chinegwundoh 2004
Dastgiri 2016
Estcourt 2016a
-
- Estcourt LJ, Fortin PM, Hopewell S, Trivella M, Hambleton IR, Cho G. Regular long‐term red blood cell transfusions for managing chronic chest complications in sickle cell disease. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD008360.pub4; CD008360] - DOI - PMC - PubMed
Estcourt 2016b
Estcourt 2017a
Estcourt 2017b
Martí‐Carvajal 2009
Martí‐Carvajal 2014
Martí‐Carvajal 2015a
Martí‐Carvajal 2016
Okusanya 2016
Oniyangi 2015
Oringanje 2016
Owusu‐Ofori 2015
References to excluded reviews
Al Hajeri 2016
Alhashimi 2010
Carson 2012
Dodd 2004
Jones 2001
Knight‐Madden 2014
Martí‐Carvajal 2012
Martí‐Carvajal 2015b
Meremikwu 1999
Nagalla 2012
Okomo 2015
Additional references
Adams 1998
-
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. New England Journal of Medicine 1998;339(1):5‐11. - PubMed
Adams 2005
-
- Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. New England Journal of Medicine 2005;353(26):2769‐78. - PubMed
Akinsheye 2011
Akinsulie 2005
-
- Akinsulie AO, Temiye EO, Akanmu AS, Lesi FEA, Whyte CO. Clinical evaluation of extract of Cajanuscajan (Ciklavit) in sickle cell anaemia. Journal of Tropical Paediatrics 2005;51(4):200‐5. - PubMed
Al‐Jaouni 2006
-
- Al‐Jaouni SK, Al‐Muhayawi SM, Qari MH, Nawas MA, Al‐Mazrooa A. Randomized clinical trial to evaluate the safety of avoiding pre‐operative transfusion in sickle cell anemia. Bahrain Medical Bulletin 2006;28(4):164‐7. []
Ansong 2013
Ballard 2017
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE Guidelines 3. rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Baum 1987
-
- Baum KF, MacFarlane DE, Maude GH, Serjeant GR. Topical antibiotics in chronic sickle cell leg ulcers. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(5):847‐9. - PubMed
Chou 2013a
-
- Chou ST. Transfusion therapy for sickle cell disease: a balancing act. Hematology 2013;2013:439‐46. - PubMed
Chou 2013b
-
- Chou ST, Jackson T, Vege S, Smith‐Whiteley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh‐matched minority donors. Blood 2013;122(6):1062‐71. - PubMed
DeBaun 2014
Expert Panel Report 2014
-
- US Department of Health and Human Services National Institutes of Health National Heart Lung and Blood Institute Expert Panel Report 2014. Evidence‐based management of sickle cell disease: Expert Panel Report, 2014. http://www.nhlbi.nih.gov/health‐pro/guidelines/sickle‐cell‐disease‐guide... (accessed 01 August 2015).
Foucan 1998
-
- Foucan L, Bourhis V, Bangou J, Merault L, Etienne‐Julan M, Salmi RL. A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. American Journal of Medicine 1998;104(4):339‐42. - PubMed
Frenette 2007
Gravitz 2014
-
- Gravitz L, Pincock S. Sickle‐cell disease. Nature 2014;515(7526):S1. - PubMed
Grosse 2011
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Green S (editors). Chapter 22: Overviews of reviews. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howard 2013
-
- Howard J, Malfroy M, Llewelyn C, Choo L, Hodge R, Johnson T, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930‐8. - PubMed
Josephson 2007
-
- Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfusion Medicine Reviews 2007;21(2):118‐32. - PubMed
Kato 2006a
Kato 2006b
Koshy 1988
-
- Koshy M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red‐cell transfusions in pregnant patients with sickle cell disease. New England Journal of Medicine 1988;319:1447–52. - PubMed
La Grenade 1993
-
- Grenade L, Thomas PW, Serjeant GR. A randomized controlled trial of solcoseryl and duoderm in chronic sickle cell ulcers. West Indian Medical Journal 1993;42(3):121‐3. - PubMed
McMahon 2010
-
- McMahon L, Tamary H, Askin M, Adams‐Graves P, Eberhardt RT, Sutton M, et al. A randomized phase II trial of Arginine Butyrate with standard local therapy in refractory sickle cell leg ulcers. British Journal of Haematology 2010;151(5):526‐24. - PubMed
Neumayr 2006
-
- Neumayr LD, Aguilar C, Earles AN, Jergesen HE, Haberkern CM, Kammen BF, et al. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. Journal of Bone and Joint Surgery 2006;88(12):2573‐82. - PubMed
NICE 2010
-
- NICE (National Institute for Health and Care Excellence). Sickle cell disease. http://cks.nice.org.uk/sickle‐cell‐disease#!backgroundsub:3. London: NICE, (accessed 01 August 2015).
Ohene‐Frempong 1999
-
- Ohene‐Frempong K. Sickle cell disease in the United Statesof America and Africa. Hematology (American Society of Hematology Education Program). American Society of Hematology 1999:64‐72.
Piel 2012
Piel 2017
-
- Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. New England Journal of Medicine 2017;376(16):1561‐73. - PubMed
Pieper 2015
-
- Pieper D, Buechter RB, Li L, Prediger B, Eikermann M. Systematic review found AMSTAR, but not R(evised)‐AMSTAR to have good measurement properties. Journal of Clinical Epidemiology 2015;68:574‐583. - PubMed
Pleasants 2014
-
- Pleasants S. Epidemiology: a moving target. Nature 2014;515(7526):S2‐3. - PubMed
Porter 2013
-
- Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. ASH Education Program Book 2013;1:447‐56. - PubMed
Rees 2010
-
- Rees DC, Williams TN, Gladwin MT. Sickle‐cell disease. Lancet 2010;376(9757):2018‐31. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Rumaney 2014
-
- Rumaney MB, Ngo Bitoungui VJ, VorsterAA, Ramesar R, Kengne AP, Ngogang J, et al. The co‐inheritance of alpha‐thalassemia and sickle cell anemia is associated with better hematological indices and lower consultations rate in Cameroonian patients and could improve their survival. PLoS ONE 2014;9(6):e100516. - PMC - PubMed
Scheunemann 2010
Schmalzer 1987
-
- Schmalzer EA, Lee JO, Brown AK, Usami S, Chien S. Viscosity of mixtures of sickle and normal red cells at varying hematocrit levels. Implications for transfusion. Transfusion 1987;27(3):228‐33. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Serjeant 1977
-
- Serjeant GR, Howard C. Isoxsuprine hydrochloride in the therapy of sickle cell leg ulceration. West Indian Medical Journal 1977;26(3):164‐6. - PubMed
Serjeant 1985
-
- Serjeant GR, Ceulaer K, Maude GH. Stilboestrol and stuttering priapism in homozygous sickle‐cell disease. Lancet 1985;2(8467):1274‐6. - PubMed
Serjeant 1997
-
- Serjeant BE, Harris J, Thomas P, Serjeant GR. Propionyl‐L‐carnitine in chronic leg ulcers of homozygous sickle cell disease: a pilot study. Journal of the American Academy of Dermatology 1997;37:491‐3. - PubMed
Shea 2007
Sparkenbaugh 2013
Steinberg 2012
Styles 2012
-
- Styles L, Wager CG, Labotka RJ, Smith‐Whitley K, Thompson AA, Lane PA, et al. Refining the value of secretory phospholipase A2 as a predictor of acute chest syndrome in sickle cell disease: results of a feasibility study (PROACTIVE).. British Journal of Haematology 2012;157(5):627‐36. - PMC - PubMed
Swerdlow 2006
-
- Swerdlow PS. Red cell exchange in sickle cell disease. Hematology 2006;2006:48‐53. - PubMed
Ubesie 2012
Vichinsky 1995
-
- Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, et al. A comparison of conservative and aggressive transfusion regimes in the perioperative management of sickle cell disease. New England Journal of Medicine 1995;333(4):206‐13. - PubMed
Wagner 2007
Wambebe 2001
-
- Wambebe C, Khamofu H, Momoh JAF, Ekpeyong M, Audu BS, Njoku OS, et al. Double‐blind, placebo controlled,randomised cross‐over clinical trial of NIPRISAN in patients with sickle cell disorder. Phytomedicine 2001;8(4):252‐61. - PubMed
Wang 2011
Ware 2012
Ware 2016
-
- Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia ‐ TCD with Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open‐label, phase 3, non‐inferiority trial. Lancet 2016;387(10019):661‐70. - PMC - PubMed
Ware 2017
-
- Ware RE, Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet 2017;390:311‐23. - PubMed
Wethers 1994
-
- Wethers DL, Ramirez GM, Koshy M, Steinberg MH, Phillips G Jr, Siegel RS, et al. Accelerated healing of chronic sickle‐cell leg ulcers treated with RGD peptide matrix. RGD Study Group. Blood 1994;84(6):1775‐9. - PubMed
Yawn 2014
-
- Yawn BP, Buchanan GR, Afenyi‐Annan AN, Ballas SK, Hassel KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence‐based report by expert panel members. JAMA 2014;312(10):1033‐48. - PubMed
Zorzela 2016
-
- Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 2016;352:i157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical