Overexpression of the Kininogen-1 inhibits proliferation and induces apoptosis of glioma cells
- PMID: 30068373
- PMCID: PMC6090912
- DOI: 10.1186/s13046-018-0833-0
Overexpression of the Kininogen-1 inhibits proliferation and induces apoptosis of glioma cells
Abstract
Background: Glioma is the most common primary central nervous system tumor derived from glial cells. Kininogen-1 (KNG1) can exert antiangiogenic properties and inhibit proliferation of endothelial cells. The effect of KNG1 on the glioma is rarely reported, so our purpose in to explore its mechanism in glioma cells.
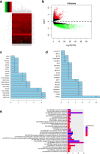
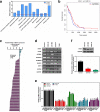
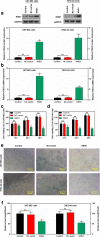
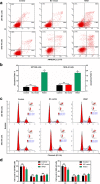
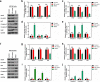
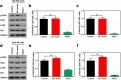
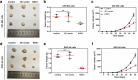
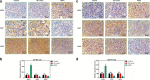
Methods: The differentially expressed genes (DEGs) were identified based on The Cancer Genome Atlas (TCGA) database. The KNG1-vector was transfected into the two glioma cells. The viability, apoptosis and cell cycle of glioma cells and microvessel density (MVD) were detected by cell counting kit-8 assay, flow cytometry and immunohistochemistry, respectively. The expression were measured by quantitative real-time PCR and Western blot, respectively. A tumor mouse model was established to determine apoptosis rate of brain tissue by terminal deoxynucleotidyl transfer-mediated dUTP nick end labeling (TUNEL) analysis.
Results: KNG1 was identified as the core gene and lowly expressed in the glioma cells. Overexpression of KNG1 inhibited cell viability and angiogenesis of glioma cells. Overexpression of KNG1 promoted the apoptosis and G1 phase cell cycle arrest of glioma cells. Moreover, the expressions of VEGF, cyclinD1, ki67, caspase-3/9 and XIAP were regulated by overexpression of KNG1. In addition, overexpression of KNG1 inhibited the activity of PI3K/Akt. Furthermore, overexpression of KNG1 decreased the tumor growth and promoted the apoptosis of decreased by overexpression of KNG1 in vivo. .
Conclusions: Overexpression of KNG1 suppresses glioma progression by inhibiting the proliferation and promoting apoptosis of glioma cells, providing a therapeutic strategy for the malignant glioma.
Keywords: Angiogenesis; Apoptosis; Glioma; KNG1.
Conflict of interest statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

