Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice
- PMID: 30068506
- PMCID: PMC6161769
- DOI: 10.1182/blood-2018-05-850859
Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice
Abstract
Inflammation is a major contributor to deep vein thrombosis (DVT). Flow restriction of the inferior vena cava (IVC) in mice induces DVT like that in humans. In this model, P-selectin-dependent adhesion of neutrophils and monocytes leads to release of neutrophil extracellular traps (NETs) and expression of tissue factor. However, it is not known what signals cause myeloid cells to generate these procoagulant effectors. Using ultrasonography and spinning-disk intravital microscopy in genetically engineered mice, we found that engagement of P-selectin glycoprotein ligand-1 (PSGL-1) and the chemokine receptor CXCR2 on rolling neutrophils propagated signals that cooperated to induce β2 integrin-dependent arrest in flow-restricted IVCs. Unlike previous reports, PSGL-1 signaling in neutrophils did not require L-selectin, and it used tyrosine 145 rather than tyrosines 112 and 128 on the adaptor Src homology domain-containing leukocyte phosphoprotein of 76 kDa. PSGL-1 and CXCR2 signaling cooperated to increase the frequency and size of thrombi, in part by stimulating release of NETs. Unlike in neutrophils, blocking PSGL-1 or CXCR2 signaling in monocytes did not affect their recruitment into thrombi or their expression of tissue factor. Our results demonstrate that neutrophils cooperatively signal through PSGL-1 and CXCR2 to promote DVT.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.P.M. is a cofounder of Selexys Pharmaceuticals, now part of Novartis AG, and of Tetherex Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures

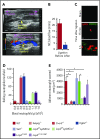
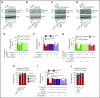
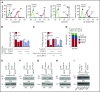

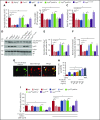

Comment in
-
Neutrophils, NETs, and immunothrombosis.Blood. 2018 Sep 27;132(13):1360-1361. doi: 10.1182/blood-2018-08-868067. Blood. 2018. PMID: 30262582 No abstract available.
References
-
- Cohen AT, Agnelli G, Anderson FA, et al. ; VTE Impact Assessment Group in Europe (VITAE). Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756-764. - PubMed
-
- Reitsma PH, Versteeg HH, Middeldorp S. Mechanistic view of risk factors for venous thromboembolism. Arterioscler Thromb Vasc Biol. 2012;32(3):563-568. - PubMed
-
- Wakefield TW, Myers DD, Henke PK. Role of selectins and fibrinolysis in VTE. Thromb Res. 2009;123(suppl 4):S35-S40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

