The nature of the DNA substrate influences pre-catalytic conformational changes of DNA polymerase β
- PMID: 30068550
- PMCID: PMC6166726
- DOI: 10.1074/jbc.RA118.004564
The nature of the DNA substrate influences pre-catalytic conformational changes of DNA polymerase β
Abstract
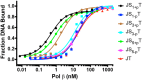
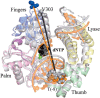
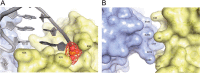
DNA polymerase β (Pol β) is essential for maintaining genomic integrity. During short-patch base excision repair (BER), Pol β incorporates a nucleotide into a single-gapped DNA substrate. Pol β may also function in long-patch BER, where the DNA substrate consists of larger gap sizes or 5'-modified downstream DNA. We have recently shown that Pol β fills small gaps in DNA during microhomology-mediated end-joining as part of a process that increases genomic diversity. Our previous results with single-nucleotide gapped DNA show that Pol β undergoes two pre-catalytic conformational changes upon binding to the correct nucleotide substrate. Here we use FRET to investigate nucleotide incorporation of Pol β with various DNA substrates. The results show that increasing the gap size influences the fingers closing step by increasing its reverse rate. However, the 5'-phosphate group has a more significant effect. The absence of the 5'-phosphate decreases the DNA binding affinity of Pol β and results in a conformationally more open binary complex. Moreover, upon addition of the correct nucleotide in the absence of 5'-phosphate, a slow fingers closing step is observed. Interestingly, either increasing the gap size or removing the 5'-phosphate group results in loss of the noncovalent step. Together, these results suggest that the character of the DNA substrate impacts the nature and rates of pre-catalytic conformational changes of Pol β. Our results also indicate that conformational changes are important for the fidelity of DNA synthesis by Pol β.
Keywords: 5′-phosphate; DNA polymerase; FRET; conformational change; crystal structure; gap size; kinetics; polymerase β.
© 2018 Huang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures






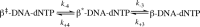
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

