Agonistic β-Klotho antibody mimics fibroblast growth factor 21 (FGF21) functions
- PMID: 30068552
- PMCID: PMC6153294
- DOI: 10.1074/jbc.RA118.004343
Agonistic β-Klotho antibody mimics fibroblast growth factor 21 (FGF21) functions
Abstract
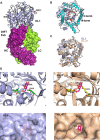
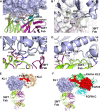
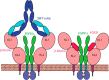
Fibroblast growth factor 21 (FGF21), an endocrine hormone in the FGF family, plays a critical role in regulating metabolic homeostasis and has emerged as a therapeutic target for metabolic diseases, including Type 2 diabetes mellitus. FGF21 functions through a receptor complex that consists of an FGF receptor (FGFR) and a co-receptor β-Klotho. Here, we identify and biochemically and structurally characterize 39F7, a high-affinity agonistic monoclonal antibody (mAb) against β-Klotho that mimics FGF21 function. The co-crystal structure of β-Klotho KL1 domain in complex with 39F7 Fab revealed that the recognition of 39F7 is centered on Trp-295 of β-Klotho in a FGF21 noncompetitive manner. KL1 adopts a (β/α)8 TIM barrel fold which resembles that of β-glycosylceramidase, but lacks molecular features for enzymatic activity, suggesting that KL1 functions as a scaffold protein instead. In vitro characterization demonstrated that, although 39F7 does not compete with FGF21, it is specific for β-Klotho/FGFR1c activation. Furthermore, the agonistic activity of 39F7 required the full IgG molecule to be bivalent, suggesting that 39F7 functions by promoting receptor/co-receptor dimerization. Supported by negative stain EM analysis of full-length β-Klotho, we propose a molecular model wherein the agonistic antibody 39F7 acts in a β-Klotho- and FGFR1c-dependent manner, mimicking FGF21 activity. More importantly, 39F7 offers promising therapeutic potential in the axis of FGF21 signaling as an antibody therapy alternative to FGF21 analogs for treatment of metabolic diseases.
Keywords: agonist; antibody; binding; crystallography; electron microscopy (EM); fibroblast growth factor (FGF); metabolism; β-Klotho.
© 2018 Min et al.
Conflict of interest statement
All authors are employees of Amgen
Figures



References
-
- Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., and Nabeshima Y. I. (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 10.1038/36285 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

