Clinical Utility of Prospective Molecular Characterization in Advanced Endometrial Cancer
- PMID: 30068706
- PMCID: PMC6279519
- DOI: 10.1158/1078-0432.CCR-18-0412
Clinical Utility of Prospective Molecular Characterization in Advanced Endometrial Cancer
Abstract
Purpose: Advanced-stage endometrial cancers have limited treatment options and poor prognosis, highlighting the need to understand genetic drivers of therapeutic vulnerabilities and/or prognostic predictors. We examined whether prospective molecular characterization of recurrent and metastatic disease can reveal grade and histology-specific differences, facilitating enrollment onto clinical trials.
Experimental design: We integrated prospective clinical sequencing and IHC data with detailed clinical and treatment histories for 197 tumors, profiled by MSK-IMPACT from 189 patients treated at Memorial Sloan Kettering Cancer Center.
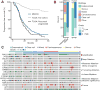
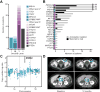
Results: Patients had advanced disease and high-grade histologies, with poor progression-free survival on first-line therapy (PFS1). When matched for histology and grade, the genomic landscape was similar to that of primary untreated disease profiled by TCGA. Using multiple complementary genomic and mutational signature-based methods, we identified patients with microsatellite instability (MSI), even when standard MMR protein IHC staining failed. Tumor and matched normal DNA sequencing identified rare pathogenic germline mutations in BRCA2 and MLH1. Clustering the pattern of DNA copy-number alterations revealed a novel subset characterized by heterozygous losses across the genome and significantly worse outcomes compared with other clusters (median PFS1 9.6 months vs. 17.0 and 17.4 months; P = 0.006). Of the 68% of patients harboring potentially actionable mutations, 27% were enrolled to matched clinical trials, of which 47% of these achieved clinical benefit.
Conclusions: Prospective clinical sequencing of advanced endometrial cancer can help refine prognosis and aid treatment decision making by simultaneously detecting microsatellite status, germline predisposition syndromes, and potentially actionable mutations. A small overall proportion of all patients tested received investigational, genomically matched therapy as part of clinical trials.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

