Anti-Folate Receptor Alpha-Directed Antibody Therapies Restrict the Growth of Triple-negative Breast Cancer
- PMID: 30068707
- PMCID: PMC6193548
- DOI: 10.1158/1078-0432.CCR-18-0652
Anti-Folate Receptor Alpha-Directed Antibody Therapies Restrict the Growth of Triple-negative Breast Cancer
Abstract
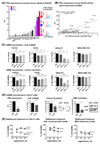
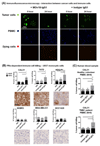
Purpose: Highly aggressive triple-negative breast cancers (TNBCs) lack validated therapeutic targets and have high risk of metastatic disease. Folate receptor alpha (FRα) is a central mediator of cell growth regulation that could serve as an important target for cancer therapy.Experimental Design: We evaluated FRα expression in breast cancers by genomic (n = 3,414) and IHC (n = 323) analyses and its association with clinical parameters and outcomes. We measured the functional contributions of FRα in TNBC biology by RNA interference and the antitumor functions of an antibody recognizing FRα (MOv18-IgG1), in vitro, and in human TNBC xenograft models.Results: FRα is overexpressed in significant proportions of aggressive basal like/TNBC tumors, and in postneoadjuvant chemotherapy-residual disease associated with a high risk of relapse. Expression is associated with worse overall survival. TNBCs show dysregulated expression of thymidylate synthase, folate hydrolase 1, and methylenetetrahydrofolate reductase, involved in folate metabolism. RNA interference to deplete FRα decreased Src and ERK signaling and resulted in reduction of cell growth. An anti-FRα antibody (MOv18-IgG1) conjugated with a Src inhibitor significantly restricted TNBC xenograft growth. Moreover, MOv18-IgG1 triggered immune-dependent cancer cell death in vitro by human volunteer and breast cancer patient immune cells, and significantly restricted orthotopic and patient-derived xenograft growth.Conclusions: FRα is overexpressed in high-grade TNBC and postchemotherapy residual tumors. It participates in cancer cell signaling and presents a promising target for therapeutic strategies such as ADCs, or passive immunotherapy priming Fc-mediated antitumor immune cell responses. Clin Cancer Res; 24(20); 5098-111. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
S.N. Karagiannis and J.F. Spicer are founders and shareholders of IGEM Therapeutics Ltd. F.O. Nestle is an employee of Sanofi US. All other authors have declared that no conflict of interest exists.
Figures




References
-
- Lehmann BD, Pietenpol JA, Tan AR. Triple-negative breast cancer: molecular subtypes and new targets for therapy. Am Soc Clin Oncol Educ Book. 2015:e31–39. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Diaz LK, Cryns VL, Symmans F, Sneige N. Triple negative breast carcinoma and the basal phenotype: From expression profiling to clinical practice. Advances in Anatomic Pathology. 2007;14:419–430. - PubMed
-
- Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

