Mesopic and dark-adapted two-color fundus-controlled perimetry in patients with cuticular, reticular, and soft drusen
- PMID: 30068928
- PMCID: PMC6292882
- DOI: 10.1038/s41433-018-0183-3
Mesopic and dark-adapted two-color fundus-controlled perimetry in patients with cuticular, reticular, and soft drusen
Abstract
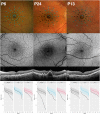
Purpose: To examine the feasibility and utility of dark-adapted two-color fundus-controlled perimetry (FCP) in patients with cuticular, reticular, and soft drusen, and to compare FCP data to microstructural spectral-domain optical coherence tomography (SD-OCT) data.
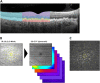
Methods: Forty-four eyes (24 eyes of 24 patients with drusen, age 69.4 ± 12.6 years; 20 normal eyes of 16 subjects, 61.7 ± 12.4 years) underwent duplicate mesopic, dark-adapted cyan and dark-adapted red FCP within 14° of the central retina (total of 12 936 threshold tests) using the Scotopic Macular Integrity Assessment (S-MAIA, CenterVue, Padova, Italy) device. FCP data were registered to SD-OCT data to obtain outer nuclear layer, inner and outer photoreceptor segment, and retinal pigment epithelium drusen complex (RPEDC) thickness data spatially corresponding to the stimulus location and area (0.43°). Structure-function correlations were assessed using mixed-effects models.
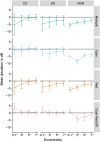
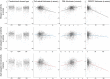
Results: Mean deviation values for eyes with cuticular, soft, and reticular drusen were similar for mesopic (-2.1, -3.4, and -3.6 dB) and dark-adapted red (-1.4, -2.6, and -3.3 dB) FCP. For the dark-adapted cyan FCP (0.1, -1.9, and -5.0 dB) and for the cyan-red sensitivity difference (+1.0, +0.5, and -2.4 dB), the mean deviation values differed significantly in dependence of the predominant drusen type (one-way ANOVA; p < 0.05). RPEDC thickness was associated with reduction of mesopic sensitivity (-0.34 dB/10 µm RPEDC thickening; p < 0.001), dark-adapted cyan sensitivity (-0.11 dB/10 µm RPEDC thickening; p = 0.003), and dark-adapted red sensitivity (-0.26 dB/10 µm RPEDC thickening; p < 0.001).
Conclusions: In contrast to mesopic FCP, dark-adapted two-color FCP allowed for meaningful differential testing of rod and cone function in patients with drusen indicating predominant cone dysfunction in eyes with cuticular drusen and predominant rod dysfunction in eyes with reticular drusen. RPEDC thickness was the strongest predictor of the evaluated SD-OCT biomarkers for point-wise sensitivity.
Conflict of interest statement
CenterVue SpA, Padova, Italy has provided research material (S-MAIA) for the conduct of this study. CenterVue had no role in the design or conduct of the experiments.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

