Double-edged sword of gonadotropin-releasing hormone (GnRH): A novel role of GnRH in the multiple beneficial functions of endometrial stem cells
- PMID: 30069003
- PMCID: PMC6070560
- DOI: 10.1038/s41419-018-0892-3
Double-edged sword of gonadotropin-releasing hormone (GnRH): A novel role of GnRH in the multiple beneficial functions of endometrial stem cells
Abstract
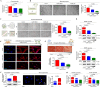
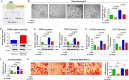
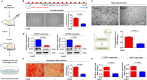
Gonadotropin-releasing hormone (GnRH) stimulates the synthesis and release of gonadotropins, which induce estrogen production and subsequent ovulation. Therefore, long-term GnRH exposure to regulate ovarian hyperstimulation is recognized as the gold standard for most in vitro fertilization (IVF) strategies. However, one of the most disappointing aspects of current IVF technology is relatively low rate (between 35 and 50%) of positive pregnancy outcomes, and the major reason for this high cancellation rate has not yet been revealed. Previous studies have demonstrated that resident stem cell deficiency limits the cyclic regenerative capacity of the endometrium and subsequently increases pregnancy failure rates. Therefore, we hypothesized that long-term GnRH exposure directly damages endometrial stem cells and consequently negatively affects pregnancy outcomes in GnRH-based IVF. In addition to their well-known roles in regulating the hypothalamus-pituitary-gonadal axis, GnRH and its receptors also localize in the extra-hypothalamic endometrium, suggesting a possible non-canonical role in endometrial stem cells. Consistent with our hypothesis, we show for the first time that GnRH suppresses the multiple beneficial functions of endometrial stem cells via the PI3K/Akt signaling pathway in vitro and in vivo. To the best of our knowledge, this is the first study to focus on the direct effects of GnRH on the regenerative potential of stem cells, and the findings will facilitate the development of more promising IVF strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

