Regulation of T cell afferent lymphatic migration by targeting LTβR-mediated non-classical NFκB signaling
- PMID: 30069025
- PMCID: PMC6070541
- DOI: 10.1038/s41467-018-05412-0
Regulation of T cell afferent lymphatic migration by targeting LTβR-mediated non-classical NFκB signaling
Erratum in
-
Author Correction: Regulation of T cell afferent lymphatic migration by targeting LTβR-mediated non-classical NFκB signaling.Nat Commun. 2019 Jun 27;10(1):2927. doi: 10.1038/s41467-019-10952-0. Nat Commun. 2019. PMID: 31249309 Free PMC article.
Abstract
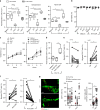
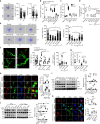
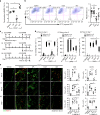
Lymphotoxin-beta receptor (LTβR) signaling in lymphatic endothelial cells (LEC) regulates leukocyte afferent lymphatic transendothelial migration (TEM). The function of individual signaling pathways for different leukocyte subsets is currently unknown. Here, we show that LTβR signals predominantly via the constitutive and ligand-driven non-classical NIK pathway. Targeting LTβR-NIK by an LTβR-derived decoy peptide (nciLT) suppresses the production of chemokines CCL21 and CXCL12, and enhances the expression of classical NFκB-driven VCAM-1 and integrin β4 to retain T cells on LEC and precludes T cell and dendritic cell TEM. nciLT inhibits contact hypersensitivity (CHS) at both the sensitization and elicitation stages, likely by inhibiting leukocyte migration. By contrast, targeting LTβR-classical NFκB signaling during the elicitation and resolution stages attenuates CHS, possibly by promoting leukocyte egress. These findings demonstrate the importance of LTβR signaling in leukocyte migration and LEC and lymphatic vessel function, and show that antagonist peptides may serve as lead compounds for therapeutic applications.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

