The Effect of Partial Premixing and Heat Loss on the Reacting Flow Field Prediction of a Swirl Stabilized Gas Turbine Model Combustor
- PMID: 30069142
- PMCID: PMC6044246
- DOI: 10.1007/s10494-017-9848-4
The Effect of Partial Premixing and Heat Loss on the Reacting Flow Field Prediction of a Swirl Stabilized Gas Turbine Model Combustor
Abstract
This work addresses the prediction of the reacting flow field in a swirl stabilized gas turbine model combustor using large-eddy simulation. The modeling of the combustion chemistry is based on laminar premixed flamelets and the effect of turbulence-chemistry interaction is considered by a presumed shape probability density function. The prediction capabilities of the presented combustion model for perfectly premixed and partially premixed conditions are demonstrated. The effect of partial premixing for the prediction of the reacting flow field is assessed by comparison of a perfectly premixed and partially premixed simulation. Even though significant mixture fraction fluctuations are observed, only small impact of the non-perfect premixing is found on the flow field and flame dynamics. Subsequently, the effect of heat loss to the walls is assessed assuming perfectly premixing. The adiabatic baseline case is compared to heat loss simulations with adiabatic and non-adiabatic chemistry tabulation. The results highlight the importance of considering the effect of heat loss on the chemical kinetics for an accurate prediction of the flow features. Both heat loss simulations significantly improve the temperature prediction, but the non-adiabatic chemistry tabulation is required to accurately capture the chemical composition in the reacting layers.
Keywords: Heat loss effects; Partial premixing; Tabulated chemistry; Turbulent combustion.
Conflict of interest statement
Compliance with Ethical StandardsThe authors declare that they have no conflict of interest.
Figures
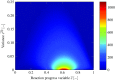


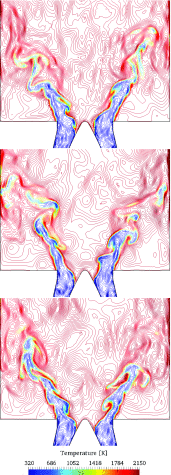


 ), perfectly premixed simulation (
), perfectly premixed simulation ( ) and partially premixed simulation (
) and partially premixed simulation ( )
)
 ), perfectly premixed simulation (
), perfectly premixed simulation ( ) and partially premixed simulation (
) and partially premixed simulation ( )
)
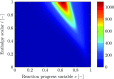
 ), perfectly premixed simulation (
), perfectly premixed simulation ( ) and partially premixed simulation (
) and partially premixed simulation ( )
)
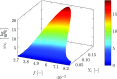
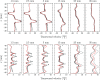
 ), perfectly premixed baseline case (
), perfectly premixed baseline case ( ) and partially premixed simulation (
) and partially premixed simulation ( ). Note that the mixture fraction is not explicitly used in the perfectly premixed simulation and the respective profile is only added as a reference
). Note that the mixture fraction is not explicitly used in the perfectly premixed simulation and the respective profile is only added as a reference
 ), perfectly premixed (
), perfectly premixed ( ) and partially premixed simulation (
) and partially premixed simulation ( )
)

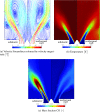

 ), perfectly premixed, adiabatic simulation (
), perfectly premixed, adiabatic simulation ( ), perfectly premixed, non-adiabatic simulation in which the chemistry is not affected by heat loss (
), perfectly premixed, non-adiabatic simulation in which the chemistry is not affected by heat loss ( ), perfectly premixed, non-adiabatic simulation (
), perfectly premixed, non-adiabatic simulation ( ) and perfectly premixed, non-adiabatic simulation including radiative heat transfer (
) and perfectly premixed, non-adiabatic simulation including radiative heat transfer ( )
)
 ), perfectly premixed, adiabatic simulation (
), perfectly premixed, adiabatic simulation ( ), perfectly premixed, non-adiabatic simulation in which the chemistry is not affected by heat loss (
), perfectly premixed, non-adiabatic simulation in which the chemistry is not affected by heat loss ( ), perfectly premixed, non-adiabatic simulation (
), perfectly premixed, non-adiabatic simulation ( ), perfectly premixed, non-adiabatic simulation including radiative heat transfer (
), perfectly premixed, non-adiabatic simulation including radiative heat transfer (
 ) and non-premixed simulation (
) and non-premixed simulation ( )
)
 ), perfectly premixed, adiabatic simulation (
), perfectly premixed, adiabatic simulation ( ), perfectly premixed, non-adiabatic simulation in which the chemistry is not affected by heat loss (
), perfectly premixed, non-adiabatic simulation in which the chemistry is not affected by heat loss ( ), perfectly premixed, non-adiabatic simulation (
), perfectly premixed, non-adiabatic simulation ( ), perfectly premixed, non-adiabatic simulation including radiative heat transfer (
), perfectly premixed, non-adiabatic simulation including radiative heat transfer ( ) and partially premixed simulation (
) and partially premixed simulation ( )
)
References
-
- Gicquel O, Darabiha N, Thevenin D. Laminar premixed hydrogen/air counterflow flame simulations using flame prolongation of ILDM with differential diffusion. Proc. Combust. Inst. 2000;28(2):1901–1908. doi: 10.1016/S0082-0784(00)80594-9. - DOI
-
- van Oijen JA, de Goey LPH. Modelling of premixed laminar flames using flamelet-generated manifolds. Combust. Sci. Technol. 2000;161(1):113–137. doi: 10.1080/00102200008935814. - DOI
-
- Gövert S, Mira D, Kok JBW, Vázquez M, Houzeaux G. Turbulent combustion modelling of a confined premixed jet flame including heat loss effects using tabulated chemistry. Appl. Energy. 2015;156:804–815. doi: 10.1016/j.apenergy.2015.06.031. - DOI
-
- Meier W, Weigand P, Duan XR, Giezendanner-Thoben R. Detailed characterization of the dynamics of thermoacoustic pulsations in a lean premixed swirl flame. Combust. Flame. 2007;150(1–2):2–26. doi: 10.1016/j.combustflame.2007.04.002. - DOI
-
- Albouze, G., Poinsot, T., Gicquel, L.: Chemical kinetics modeling and LES combustion model effects on a perfectly premixed burner, vol. 337. Combustion for aerospace propulsion (2009)
LinkOut - more resources
Full Text Sources
Other Literature Sources
