Microglia changes associated to Alzheimer's disease pathology in aged chimpanzees
- PMID: 30069930
- PMCID: PMC6283685
- DOI: 10.1002/cne.24484
Microglia changes associated to Alzheimer's disease pathology in aged chimpanzees
Abstract
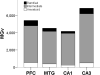
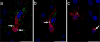
In Alzheimer's disease (AD), the brain's primary immune cells, microglia, become activated and are found in close apposition to amyloid beta (Aβ) protein plaques and neurofibrillary tangles (NFT). The present study evaluated microglia density and morphology in a large group of aged chimpanzees (n = 20, ages 37-62 years) with varying degrees of AD-like pathology. Using immunohistochemical and stereological techniques, we quantified the density of activated microglia and morphological variants (ramified, intermediate, and amoeboid) in postmortem chimpanzee brain samples from prefrontal cortex, middle temporal gyrus, and hippocampus, areas that show a high degree of AD pathology in humans. Microglia measurements were compared to pathological markers of AD in these cases. Activated microglia were consistently present across brain areas. In the hippocampus, CA3 displayed a higher density than CA1. Aβ42 plaque volume was positively correlated with higher microglial activation and with an intermediate morphology in the hippocampus. Aβ42-positive vessel volume was associated with increased hippocampal microglial activation. Activated microglia density and morphology were not associated with age, sex, pretangle density, NFT density, or tau neuritic cluster density. Aged chimpanzees displayed comparable patterns of activated microglia phenotypes as well as an association of increased microglial activation and morphological changes with Aβ deposition similar to AD patients. In contrast to human AD brains, activated microglia density was not significantly correlated with tau lesions. This evidence suggests that the chimpanzee brain may be relatively preserved during normal aging processes but not entirely protected from neurodegeneration as previously assumed.
Keywords: Alzheimer's disease; RRID: AB_223647; RRID: AB_2313890; RRID: AB_2313952; RRID: AB_2315150; RRID: AB_839504; amyloid beta protein; chimpanzee; microglia; neurofibrillary tangle; neuroinflammation.
© 2018 Wiley Periodicals, Inc.
Figures



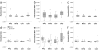
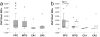

References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21(3):383–421. http://doi.org/10.1016/S0197-4580(00)00124-X - DOI - PMC - PubMed
-
- Akram A, Christoffel D, Rocher AB, Bouras C, Kovari E, Perl DP, Hof PR. Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: relationship with the progression of Alzheimer’s disease. Neurobiology of Aging. 2008;29(9):1296–1307. http://doi.org/10.1016/j.neurobiolaging.2007.03.007 - DOI - PMC - PubMed
-
- Allen JS, Bruss J, Damasio H. The aging brain: the cognitive reserve hypothesis and hominid evolution. American Journal of Human Biology: The Official Journal of the Human Biology Council. 2005;17(6):673–89. http://doi.org/10.1002/ajhb.20439 - DOI - PubMed
-
- Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Nelson PT. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathologica Communications. 2015;3(1):32. http://doi.org/10.1186/s40478-015-0209-z - DOI - PMC - PubMed
-
- Bellucci A, Bugiani O, Ghetti B, Spillantini MG. Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation. NeuroDegenerative Diseases. 2011;8(4):221–229. http://doi.org/10.1159/000322228 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

