Roflumilast enhances cisplatin-sensitivity and reverses cisplatin-resistance of ovarian cancer cells via cAMP/PKA/CREB-FtMt signalling axis
- PMID: 30069985
- PMCID: PMC6528923
- DOI: 10.1111/cpr.12474
Roflumilast enhances cisplatin-sensitivity and reverses cisplatin-resistance of ovarian cancer cells via cAMP/PKA/CREB-FtMt signalling axis
Abstract
Objective: We previously demonstrated the roflumilast inhibited cell proliferation and increased cell apoptosis in ovarian cancer. In this study, we aimed to investigate the roles of roflumilast in development of cisplatin (DDP)-sensitive and -resistant ovarian cancer.
Methods: OVCAR3 and SKOV3 were selected and the corresponding DDP-resistant cells were constructed. Cell viability, proliferation, apoptosis, cycle were performed. Expression cAMP, PKA, CREB, phosphorylation of CREB and FtMt were detected. The roles of roflumilast in development of DDP-sensitive and -resistant ovarian cancer were confirmed by xenograft model.
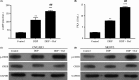
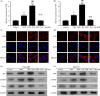
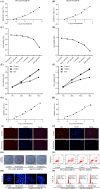
Results: Roflumilast + DDP inhibited cell proliferation, and induced cell apoptosis and G0/G1 arrest in OVCAR3 and SKOV3 cells, roflumilast induced expression of FtMt, the activity of cAMP and PKA and phosphorylation of CREB in ovarian cancer cells and the above-effect were inhibited by H89. Downregulation of CREB inhibited the roflumilast-increased DDP sensitivity of ovarian cancer cells, and the roflumilast-induced FtMt expression and phosphorylation of CREB. Also, roflumilast reversed cisplatin-resistance, and induced expression of FtMt and activation of cAMP/PKA/CREB in DDP-resistant ovarian cancer cells. Similarly, treated with H89 or downregulation of CREB inhibited the changes induced by roflumilast. In vivo, roflumilast inhibited the development of SKOV3 or SKOV3-DDP-R xenograft models.
Conclusions: Roflumilast enhanced DDP sensitivity and reversed the DDP resistance of ovarian cancer cells via activation of cAMP/PKA/CREB pathway and upregulation of the downstream FtMt expression, which has great promise in clinical treatment.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
No competing financial interests exist.
Figures










References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

