Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise
- PMID: 30070053
- PMCID: PMC6127224
- DOI: 10.1002/sctm.18-0024
Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise
Abstract
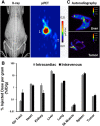
The development of mesenchymal stem cells (MSCs) as cell-based drug delivery vectors for numerous clinical indications, including cancer, has significant promise. However, a considerable challenge for effective translation of these approaches is the limited tumor tropism and broad biodistribution observed using conventional MSCs, which raises concerns for toxicity to nontarget peripheral tissues (i.e., the bad). Consequently, there are a variety of synthetic engineering platforms in active development to improve tumor-selective targeting via increased homing efficiency and/or specificity of drug activation, some of which are already being evaluated clinically (i.e., the good). Unfortunately, the lack of robust quantification and widespread adoption of standardized methodologies with high sensitivity and resolution has made accurate comparisons across studies difficult, which has significantly impeded progress (i.e., the ugly). Herein, we provide a concise review of active and passive MSC homing mechanisms and biodistribution postinfusion; in addition to in vivo cell tracking methodologies and strategies to enhance tumor targeting with a focus on MSC-based drug delivery strategies for cancer therapy. Stem Cells Translational Medicine 2018;1-13.
Keywords: Cell size; Cell-based therapy; Drug delivery; Homing; In vivo cell tracking; Mesenchymal stem cell.
© 2018 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

