Expansion Microscopy: Protocols for Imaging Proteins and RNA in Cells and Tissues
- PMID: 30070431
- PMCID: PMC6158110
- DOI: 10.1002/cpcb.56
Expansion Microscopy: Protocols for Imaging Proteins and RNA in Cells and Tissues
Abstract
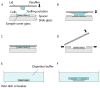
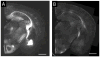
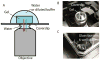
Expansion microscopy (ExM) is a recently developed technique that enables nanoscale-resolution imaging of preserved cells and tissues on conventional diffraction-limited microscopes via isotropic physical expansion of the specimens before imaging. In ExM, biomolecules and/or fluorescent labels in the specimen are linked to a dense, expandable polymer matrix synthesized evenly throughout the specimen, which undergoes 3-dimensional expansion by ∼4.5 fold linearly when immersed in water. Since our first report, versions of ExM optimized for visualization of proteins, RNA, and other biomolecules have emerged. Here we describe best-practice, step-by-step ExM protocols for performing analysis of proteins (protein retention ExM, or proExM) as well as RNAs (expansion fluorescence in situ hybridization, or ExFISH), using chemicals and hardware found in a typical biology lab. Furthermore, a detailed protocol for handling and mounting expanded samples and for imaging them with confocal and light-sheet microscopes is provided. © 2018 by John Wiley & Sons, Inc.
Keywords: FISH; antibody; confocal microscopy; expansion microscopy; fluorescence microscopy; hydrogel; imaging; immunocytochemistry; immunohistochemistry; light-sheet microscopy; super-resolution microscopy; tissue clearing.
© 2018 John Wiley & Sons, Inc.
Conflict of interest statement
ESB is co-founder of a company seeking to commercialize clinical applications of ExM. ATW, FC, PT, RG, and ESB are inventors on one or more granted or pending patents related to ExM.
Figures








References
-
- Cohen Y, Ramon O, Kopelman IJ, Mizrahi S. Characterization of inhomogeneous polyacrylamide hydrogels. Journal of Polymer Science Part B: Polymer Physics. 1992;30:1055–1067.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

