Electronic nicotine devices to aid smoking cessation by alcohol- and drug-dependent clients: protocol for a pilot randomised controlled trial
- PMID: 30071863
- PMCID: PMC6090830
- DOI: 10.1186/s13063-018-2786-1
Electronic nicotine devices to aid smoking cessation by alcohol- and drug-dependent clients: protocol for a pilot randomised controlled trial
Abstract
Background: Up to 95% of people entering treatment for use of alcohol or other drugs (AOD) smoke tobacco. Smokers receiving treatment for AOD use are interested in quitting and make quit attempts, but relapse is more common and rapid compared with the general population of smokers. New ways to address smoking in this population are needed. Electronic nicotine devices (ENDs) or electronic cigarettes hold significant potential as both cessation aids and harm reduction support. This study focuses on the potential of ENDs to facilitate smoking cessation and to sustain it in the medium term among people in treatment for AOD use. The aim of this trial is to explore the effectiveness, feasibility and acceptability of ENDs for smoking cessation compared with combination nicotine replacement therapy (NRT) for clients after discharge from a smoke-free AOD residential withdrawal service.
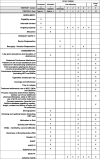
Methods/design: The study is a pragmatic randomised controlled trial. In total, 100 participants will be recruited following admission to a smoke-free residential withdrawal service in Melbourne, Australia. Participants will complete a baseline survey and be randomised to either the END group (n = 50) or the NRT group (n = 50) prior to discharge. Both groups will receive telephone counselling support from quitline. Follow-up measures will be assessed at 6 and 12 weeks following discharge. The primary outcome is continuous abstinence from smoking at 12 weeks post discharge. Secondary outcomes include: 7-day point prevalence from smoking, point prevalence abstinence from all nicotine (including NRT and ENDs), cravings and withdrawal, time to relapse, and treatment adherence (use of NRT, ENDs and quitline).
Discussion: This is the first randomised controlled trial to assess the effectiveness and acceptability of ENDs within a population dependent on AOD, a priority group with very high levels of smoking. The research will test a model of how to incorporate novel smoking cessation support into a period of high treatment receptiveness.
Trial registration: Australian New Zealand Clinical Trial Registry, ACTRN12617000849392 . Registered on 8 June 2017.
Keywords: Alcohol and other drugs; Electronic cigarettes; Nicotine replacement therapy; Smoking cessation.
Conflict of interest statement
The study was approved by Eastern Health human research ethics committee (E16–2016) and the University of Newcastle human research ethics committee (H-2017-0249). Informed consent will be obtained from all participants.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Baker A, Ivers RG, Bowman J, Butler T, Kay-Lambkin FJ, Wye P, Walsh RA, Pulver LJ, Richmond R, Belcher J, et al. Where there's smoke, there's fire: high prevalence of smoking among some sub-populations and recommendations for intervention. Drug Alcohol Rev. 2006;25:85–96. doi: 10.1080/09595230500459552. - DOI - PubMed
-
- Randall D, Degenhardt L, Vajdic CM, Burns L, Hall WD, Law M, Butler T. Increasing cancer mortality among opioid-dependent persons in Australia: a new public health challenge for a disadvantaged population. Aust N Z J Public Health. 2011;35:220–225. doi: 10.1111/j.1753-6405.2011.00682.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

