Rapid constriction of the selectivity filter underlies C-type inactivation in the KcsA potassium channel
- PMID: 30072373
- PMCID: PMC6168234
- DOI: 10.1085/jgp.201812082
Rapid constriction of the selectivity filter underlies C-type inactivation in the KcsA potassium channel
Abstract
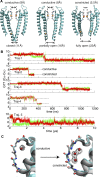
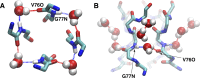
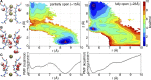
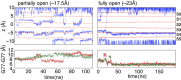
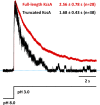
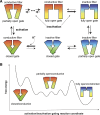
C-type inactivation is a time-dependent process observed in many K+ channels whereby prolonged activation by an external stimulus leads to a reduction in ionic conduction. While C-type inactivation is thought to be a result of a constriction of the selectivity filter, the local dynamics of the process remain elusive. Here, we use molecular dynamics (MD) simulations of the KcsA channel to elucidate the nature of kinetically delayed activation/inactivation gating coupling. Microsecond-scale MD simulations based on the truncated form of the KcsA channel (C-terminal domain deleted) provide a first glimpse of the onset of C-type inactivation. We observe over multiple trajectories that the selectivity filter consistently undergoes a spontaneous and rapid (within 1-2 µs) transition to a constricted conformation when the intracellular activation gate is fully open, but remains in the conductive conformation when the activation gate is closed or partially open. Multidimensional umbrella sampling potential of mean force calculations and nonequilibrium voltage-driven simulations further confirm these observations. Electrophysiological measurements show that the truncated form of the KcsA channel inactivates faster and greater than full-length KcsA, which is consistent with truncated KcsA opening to a greater degree because of the absence of the C-terminal domain restraint. Together, these results imply that the observed kinetics underlying activation/inactivation gating reflect a rapid conductive-to-constricted transition of the selectivity filter that is allosterically controlled by the slow opening of the intracellular gate.
© 2018 Li et al.
Figures


Comment in
-
Opening leads to closing: Allosteric crosstalk between the activation and inactivation gates in KcsA.J Gen Physiol. 2018 Oct 1;150(10):1356-1359. doi: 10.1085/jgp.201812161. Epub 2018 Aug 24. J Gen Physiol. 2018. PMID: 30143551 Free PMC article.
References
-
- Beglov D., and Roux B.. 1994. Finite representation of an infinite bulk system: Solvent Boundary Potential for Computer Simulations. J. Chem. Phys. 100:9050–9063. 10.1063/1.466711 - DOI
-
- Best R.B., Zhu X., Shim J., Lopes P.E., Mittal J., Feig M., and Mackerell A.D. Jr. 2012. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 8:3257–3273. 10.1021/ct300400x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

