Cancer immunotherapy with CAR-T cells - behold the future
- PMID: 30072559
- PMCID: PMC6334043
- DOI: 10.7861/clinmedicine.18-4-324
Cancer immunotherapy with CAR-T cells - behold the future
Abstract
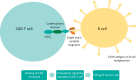
Cellular therapy is a key tool to treat haematological malignancies. Over 40,000 allogeneic and autologous haematopoietic stem cell transplants (HSCTs) are performed annually across Europe.1 Since 2017, a new T cell therapy, chimeric antigen receptor-T (CAR-T) cells have been licensed outside clinical trials. CAR-T cells have extremely potent antitumour activity, but also have a profile of toxic side effects not seen before. Cytokine release syndrome (CRS) and CAR-T cell-related encephalopathy syndrome (CRES) are common, predictable and potentially lethal side effects. Patients frequently require admission to intensive care, and management from a number of medical specialties. This exciting and powerful new therapy requires the formation of new multispecialty medical teams for safe delivery and to successfully manage the resultant complications.
Keywords: CAR-T cell related encephalopathy syndrome; CAR-T cells; cancer Immunotherapy; cellular therapy; cytokine release syndrome.
© Royal College of Physicians 2018. All rights reserved.
Figures
References
-
- Blundell J. Researches Physiological and Pathological. London: E. Cox Co. Sons; 1824.
-
- Thomas ED. A history of haemopoietic cell transplantation. Br J Haematol. 1999;105:330–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources