Soil bacterial networks are less stable under drought than fungal networks
- PMID: 30072764
- PMCID: PMC6072794
- DOI: 10.1038/s41467-018-05516-7
Soil bacterial networks are less stable under drought than fungal networks
Abstract
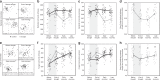
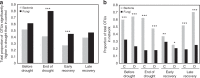
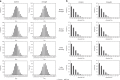
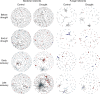
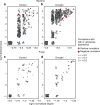
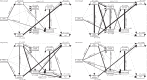
Soil microbial communities play a crucial role in ecosystem functioning, but it is unknown how co-occurrence networks within these communities respond to disturbances such as climate extremes. This represents an important knowledge gap because changes in microbial networks could have implications for their functioning and vulnerability to future disturbances. Here, we show in grassland mesocosms that drought promotes destabilising properties in soil bacterial, but not fungal, co-occurrence networks, and that changes in bacterial communities link more strongly to soil functioning during recovery than do changes in fungal communities. Moreover, we reveal that drought has a prolonged effect on bacterial communities and their co-occurrence networks via changes in vegetation composition and resultant reductions in soil moisture. Our results provide new insight in the mechanisms through which drought alters soil microbial communities with potential long-term consequences, including future plant community composition and the ability of aboveground and belowground communities to withstand future disturbances.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- De Vries FT, Wallenstein MD. Belowground connections underlying aboveground food production: a framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017;105:913–920. doi: 10.1111/1365-2745.12783. - DOI
-
- IPCC. in Climate Change 2014: Impacts, Adaptation, and Vulnerability (eds Vicente R. et al.) (Cambridge University Press, Cambridge, 2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

